hESC Transduction Protocol
Materials
24-well culture dishes (Product No. CLS3526)
Puromycin Solution (Product No. P9620)
Dispase II freshly prepared in media (Product No. D4693)
Feeder Free hESC media
Matrigel™ basement membrane
DMEM/F-12 media
MISSION® shRNA lentiviral particles
Hexadimethrine bromide (Product No. H9268)
Culture Tips
Cell Culture and Lentiviral Tips
Feeder free cultures of hESCs must be used to avoid loss of lentivirus into the feeder layer. The Stem Cell Research Center uses feeder free hESC media with Matrigel to successfully infect H1 and H9 hESC lines with both self-generated® MISSION® shRNA lentiviral particles. When the colonies are large, beginning to merge, and have centers that are dense and phase-bright compared to their edges, the hESC lines are ready to passage. This usually occurs 5–7 days after seeding, and the cells can be split 1:6 to 1:10. During the course of the transduction, cells are periodically passaged using a fresh working solution of 1 mg/mL dispase II diluted in DMEM/F-12 media. All materials that come in contact with viral material should be soaked in 10% bleach solution and disposed of carefully using biosafety level two protocols.
Protocol
Prior to Transduction — Establish the Puromycin Kill Curve
Determine the minimal puromycin concentration to kill all non-transduced cells. Use a range starting from 1–10 µg/mL of puromycin on the cells.
Transduction Procedure
DAY 0
0.1 Passage cells when 80% confluent with large colonies approximately 24 hr prior to initial transduction using dispase at 1 mg/mL.
0.2 Seed hESC cultures at a high density (1:3 is recommended) into a 24-well culture dish, taking care to maintain cells as aggregates of approximately 50–60 µm in diameter.
DAY 1 (late afternoon)
1.1 Feed hESC cells with 500 µL of pre-warmed (37 °C) mTeSR1 containing 6 µg/mL of polybrene (Hexadimethrine bromide).
1.2 Replace cultures into incubator for 15 min.
1.3 Add the desired amount of virus particles to the culture medium.
NOTE: For the initial transduction of hESC lines, Dr. Cohen’s lab uses 10 µL of 1x106 TU/mL viral particles for H1 and H9 hESC lines. Since an exact cell count cannot be determined from the previous day’s plating due to extreme aggregation of the cells (ranging from 50 to 200 cells per clump), a precise MOI cannot be calculated. The most effective amount of virus particles to be added should be determined empirically for each cell line.
1.4 Incubate for 18–20 hr at 37 °C, with 5% CO2 and 95% humidity.
DAY 2 (mid-day)
2.1 Remove medium, and replace with 500 µL of pre-warmed media supplemented with 6 µg/mL of polybrene.
2.2 Replace cultures into incubator for 15 min.
2.3 Add three times the initial amount (from Day 1) of viral particles to the culture medium.
NOTE: Dr. Cohen’s lab uses 30 µL of 1x106 TU/mL of viral particles for the secondary infection.
2.4 Incubate for 18–20 hrs at 37 °C, with 5% CO2 and 95% humidity.
DAY 3 and 4 (morning)
Remove medium daily and replace with 500 mL pre-warmed media without polybrene.
DAY 5–8
Remove media daily and replace with 500 µL pre-warmed media supplemented with puromycin. Dr. Cohen’s lab determined that a final concentration of 1 µg/mL puromycin worked best to select for successfully transduced H1 and H9 hESCs. Again, a puromycin kill curve should be run prior to any transduction experiment requiring puromycin selection.
DAY 9+
To determine the efficacy of shRNA-mediated knockdown, carry out molecular assays to quantify mRNA and protein levels. Be sure to compare to appropriate controls.
For subsequent analysis of additional time points, continue culturing hESCs with puromycin-supplemented media.
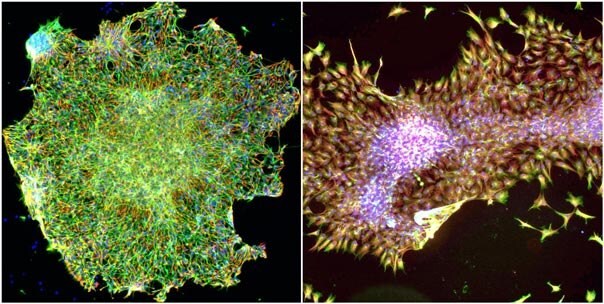
Figure 1: Feeder free hESC's were transduced using the above protocol, and then imaged by fluorescence microscopy. Nuclei were stained (blue) to indicated cell position and viability, and beta-III-tubulin (green) was detected via immunofluorescence (IF) microscopy. In addition, nestin levels (red) were also detected by IF microscopy. Left: Cells transduced with shRNA targeting RhoA resulting in differentiation into neural pre-cursors. Right: Cells transduced with non-target viral control resulting in normal stem cell colonies. Membranous staining by beta-III-tubulin and spindle morphology indicate neuronal differentiation in the RhoA shRNA treated cells. Finally, the non-target treated cells maintained strong cytoplasmic nestin staining.
Reference
To continue reading please sign in or create an account.
Don't Have An Account?