Improving Reproducibility: Best Practices for Antibodies
Introduction
In the midst of beeping lab timers, presentations and grant deadlines, it is easy to take for granted the quality of lab reagents.
Recent headlines have highlighted the risks of not validating reagents prior to experimentation, with many stories about wasted years of work, false hopes of reproducing promising results, and the destabilization of partnerships arranged with commercial entities.
One disheartening example is of a research group in Toronto who suspected an antibody to study pancreatic cancer biomarkers was faulty. After two years, $500,000 spent, and thousands of patient samples wasted on additional characterization methods, the group found that what they thought was an antibody against CUZD1 was actually against CA125.1
Despite the myriad of examples of the consequences, many labs do not validate the identity of their antibodies. Perhaps for some, validation is too time-consuming for early studies. For others, where and when to begin are key questions. What is clear, however, is that careers can suffer due to unvalidated reagents and cell lines in preliminary studies. Furthermore, pharmaceutical companies-which increasingly rely on academic discovery to develop candidates for their clinical trials-cite lack of a standard practice to validate reagents as a contributor to the reproducibility of pre-clinical studies and the subsequent high failure rate of clinical trials.
While vendors are responsible for reagent quality, researchers also share responsibility, as personal incentives for reproducible research are high. Publicity over a manuscript retraction-and the consequent loss of productivity-is much more damaging to an investigator's career than a vendor's bottom line.
Taking several small steps can reduce the risk researchers unknowingly take when running an experiment. Some of these steps are simply asking smart questions before purchasing products or recording product information as reagent boxes are opened. Other steps fit into toolboxes of validation strategies to ensure critical reagents match experimental requirements for identity, function and structure.
Antibodies
Due to the nature of the mammalian immune system, antibodies are often imperfect biological products. This imperfection often impacts specificity, selectivity and reproducibility. Today, the lack of uncharacterized antibodies and limited availability of application data has become a serious problem for the research community. While it is true that certain vendors knowingly sell mislabeled antibodies1,10, many researchers have begun overlooking the need for supporting data and as a result, they have put quality and reproducibility of their research at risk. No matter the vendor, researchers should safeguard their experiments and careers by evaluating every antibody prior to conducting their experiments. This approach reduces the risk of losing precious samples while increasing the confidence in reproducibility.
While lack of antibody specificity and selectivity may result from improper storage conditions or unexpected intricacies of the biological target an antibody’s binding characteristics may also be attributed to a number of factors related to its production and purification.
Antibodies are produced by challenging a host animal with the target antigen. Serum from that host will contain the polyclonal antibodies produced by a number of different B cells, each raising an antibody to recognize a different epitope on the antigen. Isolating single B cells from the host’s spleen and fusing them one-by-one to immortalized myeloma cells allows production of a monoclonal antibody. The single-epitope specificity of a monoclonal antibody is a result of the antibody being produced by only one B cell. While monoclonal antibodies may be more specific, any minute change in epitope structure can markedly reduce binding affinity.
Specificity is also dependent on the method used for antibody purification. Purification by Protein A or Protein G yields a less homogenous product than purification by immunogen affinity. One can also expect that the antibody provided may vary between production lots, particularly if the antibody is polyclonal.
The first step a researcher can take to help ensure the purchase of a specific and robust antibody is to carefully select an antibody vendor. A vendor’s reliability can be estimated through the availability of specification sheets and other documentation that detail characteristics about the antibody and its production process (see Box 1: Eight factors to consider when selecting an antibody vendor). Also, one should be particularly mindful of this sort of information when viewing a relatively unknown vendor’s catalog that promotes many niche antibodies that are not available from established vendors.
Since the number of applications and experiments that require quality antibodies is so vast, many vendors provide application validation data generated by third parties or external researchers (see Figures 1–4 for data examples). These third parties enhance the likelihood of successful use of the antibody since data as well as recommended titers are shared. Some of the most robust providers of data include the Human Protein Atlas project (www.proteinatlas.org), Antibody Resource (www.antibodyresource.com), Biocompare (www.biocompare.com), 1degreebio (www.1degreebio.org), and the Human Antibody Initiative (HAI) at the Human Protein Organization (HUPO), which has generated the Antibodypedia (www.antibodypedia.org) catalog of validated antibodies against human proteins.
Because an antibody’s specificity, selectivity, and reproducibility cannot be assumed from vendor specifications or third-party data, confirmatory tests are necessary. Even reputable vendors cannot account for loss of integrity during shipping or handling in the lab.2 For these reasons, careful testing, storage, and consideration of the true utility of every antibody—both upon receipt and at regular intervals during its lifetime in the lab—is crucial to safeguard the integrity of experimental results (see Box 2: Validation Techniques for Antibodies and Box 3: Tips for Storing Antibodies).
Despite the availability of evaluation or application-based data, there are no universal guidelines for the community. As part of HUPO’s Proteomic Standards Initiative, community members published a proposal to formalize standards for validating antibodies and other protein affinity reagents. The proposed Minimum Information About A Protein Affinity Reagent (MIAPAR)3 defines a checklist of product information for use by manufacturers, vendors, QC labs, users, and various databases. This checklist enables a more defined approach to compare affinity reagents and select the one most appropriate for the application.

Figure 1.Immunofluorescence. Arabidopsis suspension cells labeled with the Monoclonal Anti-Actin, clone 10-B3 (MAbGPa) (Cat. No. A0480) at a 1:100 dilution. The actin filaments were revealed with Anti-Mouse IgG-FITC (Cat. No. F6257) (green) and the nucleus is stained with DAPI (red). From M.K. Kandasamy, Genetics Department, University of Georgia, Athens, GA.

Figure 2.Immunofluorescence. Primary human osteoblast cells labeled with Anti- Vinculin (Cat. No. V9131) (red), actin was stained with Phalloidin (Cat. No. P5282) (green) and nuclei stained blue. From Eng San Thian, Materials Science and Metallurgy, University of Cambridge, Cambridge, United Kingdom.
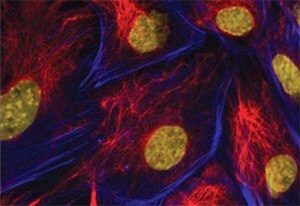
Figure 3.Immunofluorescence. Imna(-/-) mouse embryonic fibroblasts stained with Monoclonal Anti-Vimentin, clone LN-6 (Cat. No. V2258) at a 1:40 dilution (red), Anti-Actin (blue) and DAPI (yellow). From Shyam Khatau, Department of Chemical and Biomolecular Engineering, Johns Hopkins University, Baltimore, MD.
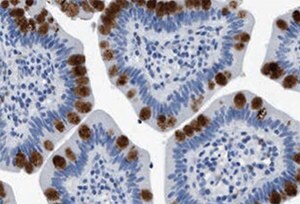
Figure 4.Immunohistochemistry. Anti-HABP2: Cat. No. HPA019518: Immunoperoxidase staining of formalin-fixed, paraffin-embedded human small intestine shows strong intracellular proteinaceous material positivity in glandular cells.
BOX 1: Eight Questions to Consider When Selecting an Antibody Vendor
Asking a few simple questions of your vendor before purchasing an antibody can help differentiate reliable suppliers. A reputable vendor will be willing to answer the following:
- Can you provide documentation of the antibody titer, immunogen or epitope sequence, and various validated applications?
- Are references and journal citations available for the antibody?
- What are the names of positive and negative cell line controls?
- How was the antibody purified? While the choice of purification method does not necessarily correlate with the quality of the antibody, different methods are useful for purifying monoclonal and polyclonal antibodies. For monoclonal antibodies, Protein A/G purification is sufficient. For polyclonal antibodies, affinity purification may be used as an alternative to Protein A/G purification.
- Is the supporting antibody performance data reflective of a native or recombinant antigen? For Western blot data, the vendor should show the entire gel, not just the band of interest. Ideally, multiple cell lines should be analyzed.
- Was the antibody raised in-house or brought in from an external supplier? If the antibody was brought in from an external supplier, is the vendor willing to share the identity of their supplier or how externally-sourced antibodies are qualified?
- Are data for antibody performance for the indicated applications available? If so, are the relevant protocols provided? If not, does the vendor have a guarantee program that gives researchers one year to self-validate the antibody and return it if there is an issue?
- Is technical support available to answer specific questions about antibody properties and purification?
BOX 2: Techniques for Evaluating Antibodies
Performing a Western blot is the simplest first step to evaluate a new antibody before use. If the end application is not a Western blot, the antibody must be tested for that application, whether it is ELISA, immunohistochemistry, immunoprecipitation, or another technique.
The frequency of antibody evaluation depends on the clonality of the antibody. Monoclonal antibodies are considered highly specific, premium reagents that generate the most reproducible results, and thus may only require testing before the first use. Polyclonal antibodies have a higher batch-to-batch variation and therefore require that every lot of material be evaluated. If a new lot of material fails to perform comparably to a previous lot, first reach out to the vendor to see if they have made any small changes, such as immunogen sequence, and for technical advice if necessary.
Western Blot
For Western blots, use a panel of positive and negative cell lines with variable expression levels of the protein of interest. If such lines do not exist, transfect the protein of interest in non-expressing cells to create a positive control or use RNAi to knock down the protein of interest to generate a negative control.
It is important to assess the final blot for multiple bands. A monoclonal antibody and a pure polyclonal antibody should ideally produce only one band for the protein of interest. In some cases, these antibodies will produce multiple lighter bands in addition to the band of interest. Compare these bands with the vendor’s full western blot image. If the band of interest is not present at several concentrations, consult vendor and/or discard antibody.
A pattern of bands is not necessarily the sign of a faulty antibody, but could indicate that the target protein is expressed in multiple isoforms or undergoes post-translational modifications that also interact with the antibody. Multiple lower molecular weight bands may also indicate cell lysate degradation. For more sensitive applications, multiple bands may indicate that a monoclonal or highly specific antibody is required.
Average time: 4 hours Average cost: $100 to $400
Mass Spectrometry & Capillary Electrophoresis
When precision characterization is necessary for specific applications (e.g. biomarker validation or therapeutic development), liquid chromatography combined with mass spectrometry detection (LC-MS) can be used to analyze the structural composition of a monoclonal antibody. Features such as molecular weight, amino acid sequence, post-translational and other modifications, carbohydrate structure, and disulfide linkages can be interrogated via methods such as sub-unit mapping, peptide mapping, N-terminal sequencing and glycan profiling. Capillary electrophoresis based methods can also provide data detailing the purity, molecular weight, isoelectric point and charge heterogeneity of a purified antibody preparation.
For the average researcher, these techniques are usually not required.
Average time: 14–21 days (from a typical service provider)
Average cost: $1,500 to $3,500
BOX 3: Tips for Storing and Using Antibodies
Antibody performance can degrade significantly over time and when subjected to improper storage conditions. In addition to recommendations from the vendor, the practices below help to ensure maximum antibody performance and longevity.
Storage
- Aliquot antibodies (e.g., 20 μL) to avoid contamination and minimize free/thaw cycles, particularly for extended storage. Some antibody formulations that contain glycerol, BSA, or other protein stabilizers may tolerate repeated freeze/thaws cycles, but it is best to avoid unnecessary temperature changes.
- Do not store antibodies in “frost-free” freezers.
- If slight turbidity occurs after prolonged storage, clarify the solution by centrifugation before use.
- For continuous use, store antibodies at 2–8 °C for up to one month.
- Monitor freezer temperature and maintain a temperature log. Program an alert to sound if the temperature goes above or below desired thresholds.
- To maximize sample recovery, use low-binding tubes when working with or storing antibodies.
- When opening a new supply of antibodies, record the open date, lot/batch number, product number, expiry date, aliquot labels, and any special instructions in a lab notebook. Without this information, doubts or mistakes at a later cannot be addressed.
Usage
- To increase confidence in antibody performance, Sigma-Aldrich recommends lot-by-lot validation of polyclonal antibodies. Monoclonal antibodies exhibit greater lot-to-lot consistency, but validation should still be conducted for each lot.
- Keep antibodies on ice when working at the bench.
- To obtain the best results, first determine the optimal working concentration of the antibody for your application by a titration test.
- Working dilution samples should be discarded within 12 hours of preparation.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?