Column Packing and Preparation for Size Exclusion Chromatography
A well-packed column is essential for a high-resolution fractionation on any SEC medium. Prepacked columns from Cytiva will ensure reproducible results and the highest performance. If the column volume or medium you require is not available as a prepacked column, contact your local Cytiva sales representative to inquire about our column packing services.
Packing a column is a very critical stage in any SEC experiment. A poorly packed column will give rise to uneven flow, peak broadening, and loss of resolution. It can also affect achievable flow rates. If you decide to pack an SEC column yourself then the guidelines in this appendix will apply at any scale of operation.
An instructive video on a CD, is available to demonstrate how to produce a well-packed column (Ordering information for Column Packing to The Movie). It focuses particularly on the importance of column packing for gel filtration. SEC is simple to perform once a well-packed column has been obtained. Providing that a column is used and maintained carefully it can be expected to give reproducible, high-resolution results for a long time.
Ensure that there is sufficient buffer for long, unattended runs or that the pump is programmed to stop the flow after a suitable time. SEC columns that run dry must be repacked.
Columns for packing SEC media
Empty columns from Cytiva are fully compatible with the high flow rates achievable with modern media and a broad range of column dimensions is available. Ordering information for empty columns and main accessories can be found at the back of this handbook.
Adapters are adjustable column end pieces that help to eliminate any disturbances to the surface of the packed medium as sample is applied and to prevent insoluble particles from entering and blocking the column.
Tricorn and XK empty columns are delivered with one adapter, but a second adapter can be used instead of a column end piece if a shorter bed height is required. HiScale™ columns are equipped with dual adapters. A range of accessories are available for all empty columns.
Longer columns (50 cm and more) can be difficult to pack under normal laboratory conditions. As alternatives, use our column packing services or connect two or more shorter columns (20 or 30 cm bed height) in series to achieve the required bed height.
Checking column efficiency
Column performance should be checked at regular intervals by determining the theoretical plate number and peak symmetry. Note that the result for column efficiency is dependent on the system used, including the capillaries and dead volumes. This means that the column efficiency given in the specification for the column (tested on another system) will not be the same as your initial column efficiency result.
Typical values for column performance:
Superdex prep grade: Efficiency N/m > 10 000, Peak symmetry As = 0.70 to 1.30 Sephacryl HR: Efficiency N/m < 9000, Peak symmetry As = 0.80 to 1.50
- 1. Equilibrate the packed column in distilled water at the recommended flow rate given in the instructions.
- Inject acetone (20 mg/mL in water) in a volume equivalent to 0.2% of the total packed column volume.
- Monitor UV absorbance 280 nm from the time of injection until the acetone peak has eluted and the signal has returned to baseline.
- Calculate column efficiency, that is, the number of theoretical plates per meter (N/m):
N/m = 5.54 (Ve / W1/2)2/L
where
Ve = peak elution (retention) volume
W1/2 = peak width at half peak height
L = bed height (m)
Ve and W1/2 are in same units
Calculate the asymmetry factor (As):
As = b/a
where
a = first half peak width at 10% peak height
b = second half peak width at 10% peak height

Figure A1.1 Determination of column efficiency by number of theoretical plates and peak symmetry.
Column packing for high-resolution fractionation using Superdex prep grade and Sephacryl High Resolution
Superdex prep grade and Sephacryl High Resolution media should be packed and equilibrated at high flow rate using a column from the XK-series. XK columns are optimally designed for SEC
with a bed design that ensures a uniform liquid flow and a dead space at the column outlet of less than 0.1% of the column volume in order to minimize dilution and to prevent remixing of separated peaks. XK columns are manufactured from materials that do not interfere with labile biological substances. They are easy to dismantle and reassemble for thorough cleaning, which is particularly important when handling biological samples.
Ensure that the column and all components are clean and in good condition. It is particularly important that the nets, net fasteners, and glass tube are not damaged. Use well degassed buffers and equilibrate all materials to the temperature at which the separation will be performed. Avoid columns with large dead volumes as this will affect resolution.
For high-resolution fractionation, use bed heights between 30 and 60 cm. Apply sample volumes equivalent to between 1% and 2% of the column volume. The sample volume can be increased up to 4% if resolution in the particular application is good enough.
The settled medium should have a volume of 1.15-fold that of the required packed column volume, Table A1.1 to A1.3 for examples.
- Superdex prep grade and Sephacryl HR are supplied swollen in a suspension containing 20% ethanol as a preservative. Suspend the medium by shaking gently and pour a sufficient quantity into a graduated glass cylinder or beaker.
Avoid using magnetic stirrers, spatulas or glass rods since they can damage the matrix. - Wash the medium with 5 to 10 column volumes of distilled water on a glass filter and resuspend in distilled water to a final concentration of 50% settled medium. The medium must be thoroughly washed to remove the 20% ethanol storage solution. Residual ethanol can interfere with subsequent procedures.
To produce a more evenly dispersed slurry of Superdex prep grade, Tween™ 20 (250 mL per 500 mL washed slurry) can be added in order to reduce surface tension. - Wet the bottom filter by injecting distilled water through the effluent tubing. Close the end piece outlet. Mount filter and bottom end piece onto the column.
- Attach the packing reservoir tightly to the column.
For XK 16 and XK 26 columns using a second column instead of a packing reservoir makes it easier to obtain a well-packed column. The second column is used with Packing Connector XK 16 or XK 26 as appropriate. - Mount the column and packing reservoir vertically on a laboratory stand.
- Fill the column with distilled water to a height of 2 cm above the column end piece.Avoid air bubbles.
- Degas the suspended medium under vacuum and carefully pour the suspended medium down the wall of the column using a glass rod. Avoid introducing air bubbles. Pour everything in a single operation and fill the reservoir to the top with distilled water.
- Connect the pump outlet to the inlet on the packing reservoir. Open the column outlet and start the flow of buffer, Table A1.4 for flow recommendations.
To achieve satisfactory column efficiency, Superdex prep grade must be packed ntwo steps: Step 1 for 2 h or until the bed has reached a constant height and Step 2for 60 min. Table A1.4 shows the flow rates for each step.
Sephacryl HR can usually be packed satisfactorily using only the higher flow rate given in Step 2 of Table A1.4. Use the two step process if the column efficiency was unsatisfactory after the first attempt. - Stop the pump and remove the packing reservoir. Carefully fill the column with distilled water to form an upward meniscus at the top and insert the adapter. Adjust the adapter to the surface of the packed bed.
- Continue packing the column at the flow rate used in Step 2 for approximately 10 min. If the recommended flow rate cannot be obtained, use the maximum flow rate the pump can deliver. Mark the position of the top of the packed medium, stop the pump, close the column outlet, move the adapter down onto to the surface of the medium and then push the adapter a further 3 mm into the medium. The column is now ready to use. Table A1.4 for maximum recommended flow rate and operating pressure for Sephacryl HR and Superdex prep grade media.
Do not exceed maximum pressures during packing: 0.2 to 0.4 MPa, 2 to 4 bar for Sephacryl S-300 HR and S-100 HR respectively, 0.15 MPa, 1.5 bar for Sephacryl S-400 HR and S-500 HR, and 0.4 to 0.5 MPa, 4 to 5 bar for Superdex prep grade (75 and 200). Always check the specific storage instructions supplied with the product.
Column packing for group separations using Sephadex
Sephadex is supplied as a dry powder and must be allowed to swell in excess buffer before use. After swelling, adjust with buffer to form a thick slurry from which air bubbles are removed under vacuum. Approximately 75% settled medium is suitable. Fine particles can be decanted.
Accelerate the swelling process by using a boiling water bath (Table A1.5). This also serves to degas the suspension. Allow the suspension to cool before use.
Ensure that the column and all components are clean and in good condition. It is particularly important that the nets, net fasteners, and glass tube are not damaged. Use well-degassed buffers and equilibrate all materials to the temperature at which the separation will be performed. Keep a packed column away from locations that are exposed to drafts or direct sunlight that can cause temperature changes and the formation of bubbles.
For group separations, use up to 10 cm bed height. Sample volumes can be up to 30% of the column volume. Pack a quantity of medium up to five-fold the volume of the sample to be desalted.
Note: These instructions assume that a column with two adapters is used for packing.
- Weigh out the correct amount of dry Sephadex and allow the medium to swell according to the instructions above. Avoid using magnetic stirrers, spatulas, or glass rods since they can damage the medium.
- Wet the bottom filter by injecting distilled water through the effluent tubing. Close the end piece outlet. Mount filter and bottom end piece onto the column.
- If the slurry volume is greater than the volume of the column, attach a packing reservoir to the column.
- Mount the column and packing reservoir vertically on a laboratory stand.
- Fill the column with distilled water or buffer to a height of approximately 2 cm above the column end piece. Avoid air bubbles.
- Pour the well-mixed and well-degassed suspension in a single operation down the inside wall using a glass rod. Avoid introducing air bubbles.
- Connect the pump outlet to the inlet of the packing reservoir. Open the column outlet and start the flow of buffer. Pass 2 to 3 column volumes of buffer through the column in order to stabilize the bed and equilibrate completely. Use a slightly higher flow rate than the flow rate to be used during separations.
- Maintain the packing flow rate for at least 3 column volumes after a constant bed height is obtained.
- Mark the bed height on the column and close the column outlet. Remove the packing reservoir.
- Add buffer carefully to fill the column and form an upward meniscus.
- Connect all tubings. Slacken the adapter tightening mechanism and insert the adapter at an angle into the column so that no air is trapped under the net. Slide the adapter slowly down the column until the mark is reached. Note that the outlet of the adapter should be open and the column outlet should be closed.
- Adjust the tightening mechanism to give a sliding seal between the column wall and O-ring. Screw the adapter onto the column.
- Continue packing the column for approximately 10 min. Stop the pump, close the column outlet, and move the top adapter down onto the surface of the medium. Push the adapter a further 3 mm into the medium. The column is now ready for equilibration.
Sephadex G-10, G-25, and G-50 obey Darcy’s law, that is, if the flow rate is doubled then the column pressure will double, hence maximum values for flow rates or operating pressures do not need to be considered (Appendix 2 for an explanation of Darcy’s law).
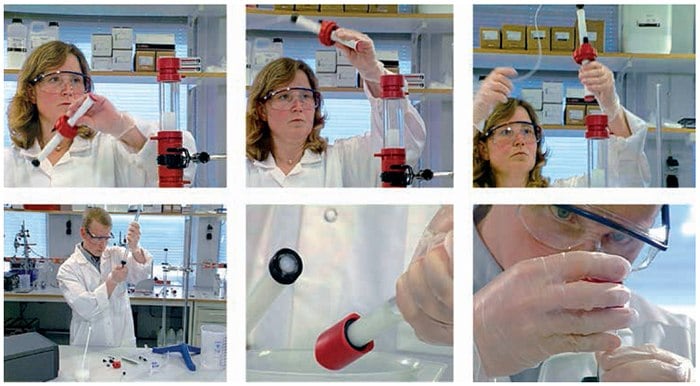
Figure A1.2Column Packing – The Movie provides a step-by-step demonstration of column packing.
Controlling flow rates
The safest and easiest way in which to control flow rates during column packing and chromatography separation is to use a pump controlled within an ÄKTA chromatography system. Accurate and reproducible flow control is particularly important for efficient column packing and when repeating experiments or performing routine preparative work. A peristaltic pump can be used with Sephadex packed in smaller columns.
The maximum flow rate achievable will depend on column diameter and buffer viscosity. Narrow columns allow a higher pressure and higher linear flow (cm/h) than wide columns.
Always connect a pump so that buffer is pumped onto the column (rather than connecting the pump after the column and drawing buffer through the column). This reduces the risk of bubble formation due to suction effects.
Always use a flow rate for column packing that is higher than the flow rate used for separation.
Do not exceed the maximum recommended values for pressure or linear flow for the medium. Exceeding these values might cause the medium to compress and reduce the flow rate and resolution during the separation.
Do not exceed 75% of the packing flow rate during any separation.
Do not use a peristaltic pump when packing Superdex or Sephacryl media in larger columns since a flow rate high enough to obtain high-resolution fractionation cannot be achieved.
Materials
如要继续阅读,请登录或创建帐户。
暂无帐户?