Cyclodextrins
Many metabolically important compounds, such as lipid-soluble vitamins and hormones, have very low solubilities in aqueous solutions. Various techniques have been used to solubilize these compounds in tissue culture, cell culture, or other water-based applications. A frequently used approach is to use cyclodextrin as a “carrier” molecule to facilitate the dissolution of these compounds.
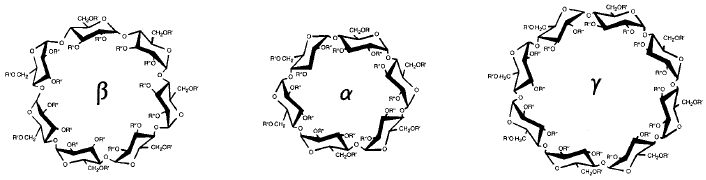
Structural representations of β-cyclodextrin, α-cyclodextrin, and γ-cyclodextrin. The cyclodextrins are cyclic oligosaccharides consisting of 7, 6, or 8 (respectively) glucopyranose units.
The solubility of natural cyclodextrins is very poor and initially this prevented cyclodextrins from becoming effective complexing agents. In the late 1960’s, it was discovered that chemical substitutions at the 2-, 3-, and 6-hydroxyl sites would greatly increase solubility. The degree of chemical substitution and the nature of the groups used for substitution determine the final maximum concentration of cyclodextrin in an aqueous medium. Most chemically modified cyclodextrins are able to achieve a 50% (w/v) concentration in water.
Cavity size is the major determinant as to which cyclodextrin is used in complexation. “Fit” is critical to achieving good incorporation of cyclodextrins. α-Cyclodextrins have small cavities that are not capable of accepting many molecules. γ-Cyclodextrins have much larger cavities than many molecules to be incorporated, and cyclodextrin hydrophobic charges cannot effectively interact to facilitate complexation. The cavity diameter of β-cyclodextrins has been found to be the most appropriate size for hormones, vitamins, and other compounds frequently used in tissue and cell culture applications. For this reason, β-cyclodextrin is most commonly used as a complexing agent.
Hydrophobic molecules are incorporated into the cavity of cyclodextrins by displacing water. This reaction is favored by the repulsion of the molecule by water. This effectively encapsulates the molecule of interest within the cyclodextrin, rendering the molecule water-soluble. When the water-soluble complex is diluted in a much larger volume of aqueous solvent, the process is reversed, thereby releasing the molecule of interest into the solution.
Our product line of water-soluble complexes includes cyclodextrins and soluble cyclodextrin-complexes of biochemicals commonly used in tissue and cell culture applications.
Materials
To continue reading please sign in or create an account.
Don't Have An Account?