Extracellular Matrix Proteins and Tools for Cell Culture Optimization
What is the Extracellular Matrix (ECM)?
Animal cells and tissue culture techniques are constantly improved to optimize in vitro cell culture conditions. Extracellular Matrix (ECM) protein coatings, chemical or physical modification of the cell culture vessel, have proven to be efficient methods to better mimic in vivo cell behavior. We describe here the different coatings available, with some new technologies highlights.
In 1900’s, animal tissues were cultured on glass surfaces, but as they require careful cleaning procedures, researchers started experimenting with disposable plastic culture vessels made up of polystyrene.1,2 However, plastic culture vessels have a certain number of limitations:3
- Difficulty in cell growth and cell attachment in serum-free media
- Change in cell shape, polarity and morphology
- Increase cell proliferation and decreased differentiation
- Less reactive to hormones and growth factors
Researchers then began coating vessel with both biological materials (biological coating) and synthetic polymers (chemical coating) that can enhance cell attachment, growth and differentiation. The growth of cells on coated surfaces is a more relevant representation of natural environment as contrary to the growing cells on flat, 2D plastic surfaces. This technique can be qualified as Physiological 2D environment, or 2.5D cell culture condition.
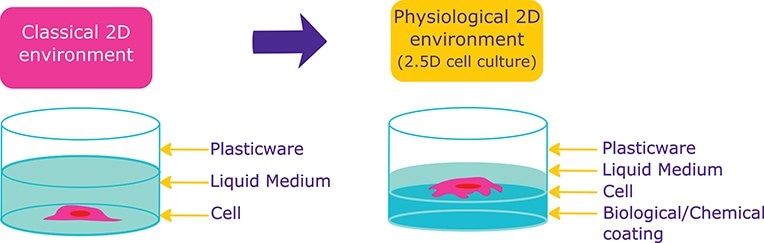
Figure 1.Cells in presence of Extracellular matrix proteins undergo physiologically relevant behaviours than cells in 2D conditions.
Cell cultured in presence of biological (extracellular matrix proteins) or chemical (Poly-Lysine…) coating are undergoing more physiologically relevant behaviors than cells cultured in classical, 2D cell culture conditions.
ECM Proteins
Tissues are not just tightly packed with cells; most of the volume contains extracellular space and is filled with complex meshwork of proteins called the extracellular matrix (ECM). The components of ECM in most tissues are secreted by fibroblasts and are categorized into proteoglycans and fibrous proteins (Collagen, gelatin, fibronectin and laminin).4 These components give structural support and facilitate cellular communication. Integrins, the transmembrane proteins on the cell surface that link the cytoskeleton of cells to the ECM, activate signaling pathways that regulate cell proliferation, morphology, adhesion and cell death.
Laminin
Laminin is major component of basal lamina. It is composed of three long polypeptide chains (designated α, β, and γ) held together by disulfide bonds and arranged in asymmetric cross shape. Laminin acts as glue, which holds cells and ECM together. It has active domains for collagen binding, cell adhesion, heparin binding and neurite outgrowth fragment. Laminin modulates cell growth, motility and signaling pathways3,8.
Fibronectin
Fibronectin is a large glycoprotein (220 kDa) composed of two polypeptide chains (dimer) joined by disulfide bonds at one end. Each polypeptide is further folded into functionally and structurally distinct domains which bind to various components of ECM (glycosaminoglycans, proteoglycans, and collagen) and cell surface proteins. Fibronectin is secreted by wide variety of connective tissue cells, including: fibroblasts, chondrocytes, schwann cells, macrophages, intestinal epithelial cells and hepatocytes7.
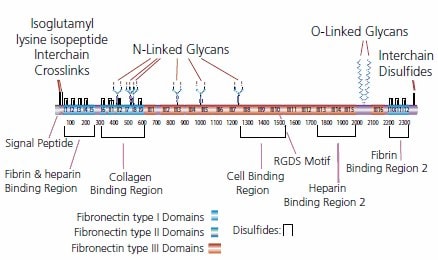
Figure 2. Structure of fibronectin.
Fibronectin is a multifunctional protein involved in cell adhesion and spreading. It also regulates cellular morphology, cell migration, cytoskeletal organization, hemostasis and wound repair.
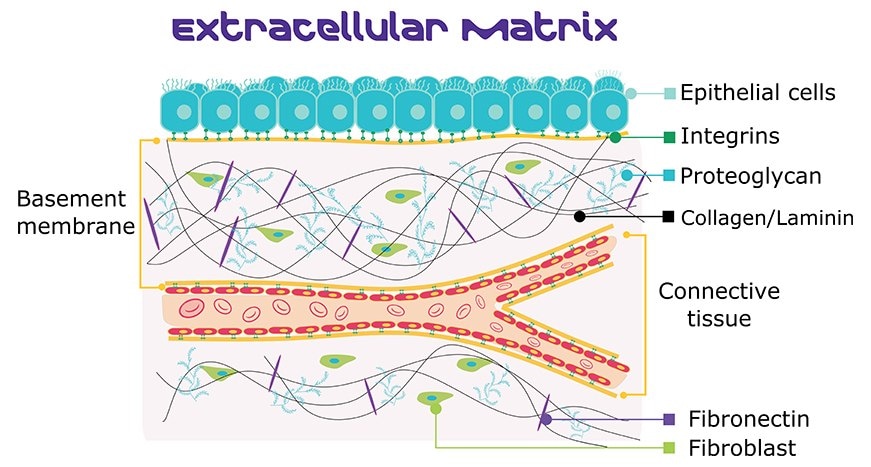
Figure 3. Basement membrane structure, including ECM proteins laminin, collagen, and fibronectin.
Collagen
Collagen is the most abundant protein in mammals, constituting 25% of total protein mass. It is composed of three polypeptide chains (designated alpha chains) arranged in a helical conformation, rich in glycine and proline residues. There are more than 20 different types of collagens, of them collagen I, II, III, V and XI are fibrillar collagen commonly found in connective tissue. Collagen type IX and XII are fibril-associated collagen, which link fibrils to one another and to the components of extracellular matrix. While collagen type IV and VII are network-forming collagen that constitutes major part of basal lamina.5
In tissues, collagen provides structural support, strength and resilience, and in cell culture it is used to study growth, differentiation and migration of cells6.
Gelatin
Gelatin is a heterogeneous mixture of water-soluble proteins of high average molecular masses, that is present in collagen. The proteins are extracted by boiling the relevant skin, tendons, ligaments, and bones in water. Type A gelatin is derived from acid-cured tissue, while type B is derived from lime-cured tissue.
Gelatin from porcine skin, referred to as type A gelatin, is generated from the acidic digestion of collagen and is mainly comprised of majorly glycine, proline and hydroxyproline. This gelatin displays a random coil structure after digestion from the triple helical collagen, differing from type B bovine gelatin at the N-terminal sequence.
Gelatin is commonly used to improve the attachment of cells and coating of cell culture plates or dishes used for embryonic stem cell culture, testicular cell culture, and neural rosettes.
Vitronectin
Vitronectin is glycoprotein of 459 aminoacids, found in ECM and blood. It circulates in blood either in the form of single chain moiety of 75kDa or as two chain moiety of 65kDa and 10kDa. Vitronectin interacts with polysaccharides (Glycosaminoglycans) and proteoglycans, acting as cell adhesion molecule. Although, vitronectin and fibronection have similar functions and have an Arg-Gly-Asp cell recognition sequence, they are structurally and immunologically distinct.9
Vitronectin acts as inhibitor of cytolytic complement pathway and have physiological role in coagulation pathway. In addition, it promotes cell migration, proliferation, differentiation and spreading of endothelial and neoplastic cells.
Chemical and Synthetic Coatings: Poly-Lysine and Poly-Ornithine
Coating of synthetic polymers (poly amino acids) facilitates the attachment of both cells and proteins. Poly-amino acids like poly-lysine and poly-ornithine create a positive charge on polystyrene and increase the positively-charged sites available for cell binding.11 They are also used in combination with attachment factors which can promote electrostatic interaction between negatively-charged ions on the cell membrane and positively-charged ions of attachment factors on the culture surface.
Ready-to-use, Pre-Mixed Attachment Factor Solutions
Our ready-to-use attachment factor mix solutions are designed to coat cell culture flasks and plates, promoting the attachment, differentiation, and proliferation of variety of cell types where adhesion is dependent upon ECM-coated surface. Conventional cell culture methods involve time-consuming preparation steps, including coating plates with various attachment factors, which can extend the overall assay timeline. These attachment factor mix solutions help simplify cell culture preparation, reduce end-user optimization, minimize the number of steps and time needed, and cut down on washing reagents. Researchers can also initiate their assays on the same day as plate coating, eliminating the need for overnight waiting before applying a second attachment factor. The working concentration of the attachment factor mix is pre-optimized for use in a 10 x 96-well plate, eliminating the need for dilution.
Attachment Factor Mix Applications
The combination of Laminin with poly-l-lysine (PLL), poly-l-ornithine (PLO), or poly-d-lysine (PDL) is often used to culture and promote epithelial and neuronal cell attachment and differentiation. These mixes can be stored at room temperature and are more stable compared to Laminin on its own, which requires storage at -20˚C and is prone to denaturation with repeated freeze-thaw cycles. When cells can digest poly-L-lysine, it is recommended to use the Laminin with Poly-D-lysine mix, to prevent excessive uptake of L-lysine.
To validate the effectiveness of the ready-to-use Laminin mixes, neural stem cells (NSCs) were cultured using two coating methods for comparison. In the standard method, PLL/PLO/PDL and Laminin were incubated together, followed by a wash and a second overnight incubation. Using our new simplified method, PLL (LPLL001), or PLO (LPLO001), or PDL (LPDL001) were incubated with the pre-mixed Laminin coating Solution for a 1-hour incubation at 37°C. The results show NSC attachment and proliferation using the ready-to-use Coating Solution Mixes (Figures 4-6).
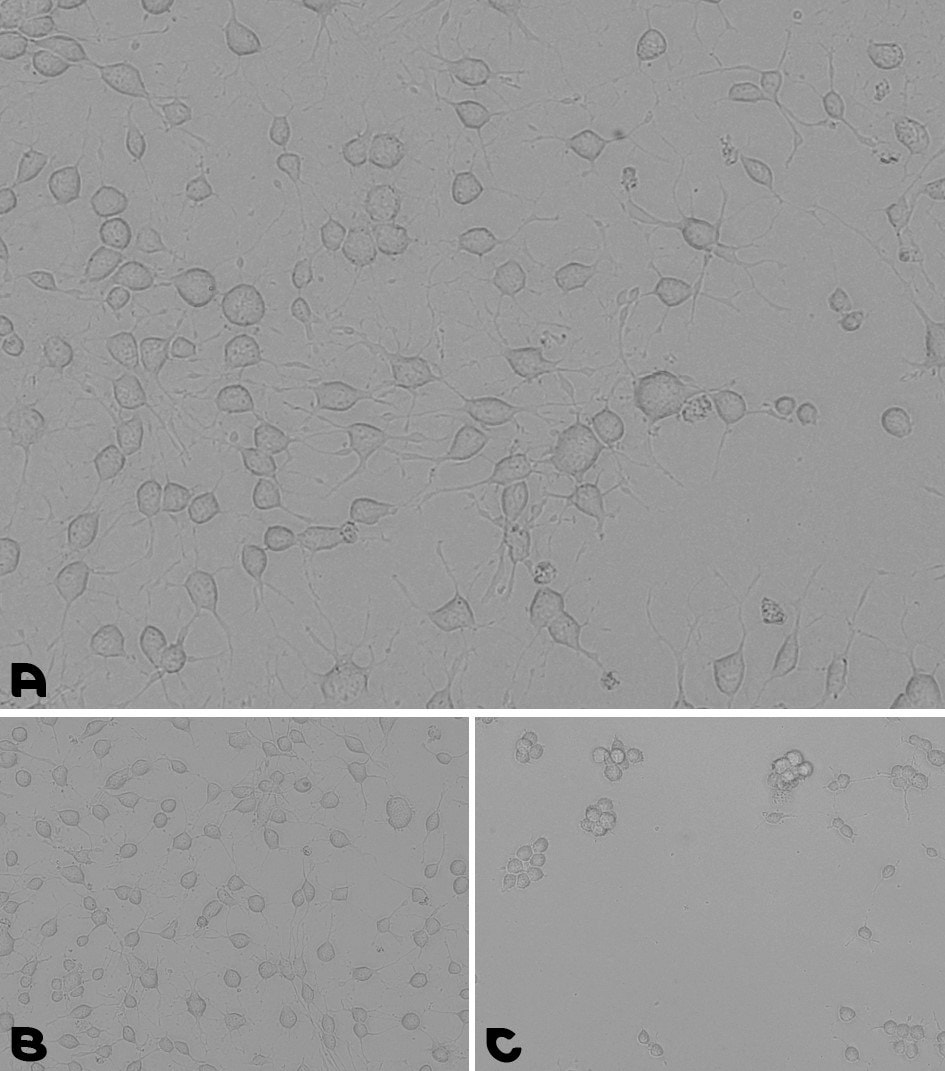
Figure 4.Neural stem cells culturing assay. A. Coating prepared by a 1-hour incubation at 37°C with the pre-mixed coating Solution of Laminin with PDL. B. Coating prepared by an overnight incubation with PDL, followed by a wash and a second overnight incubation with Laminin. C. NSCs were cultured for 48 hours on a TC plate without any coating.
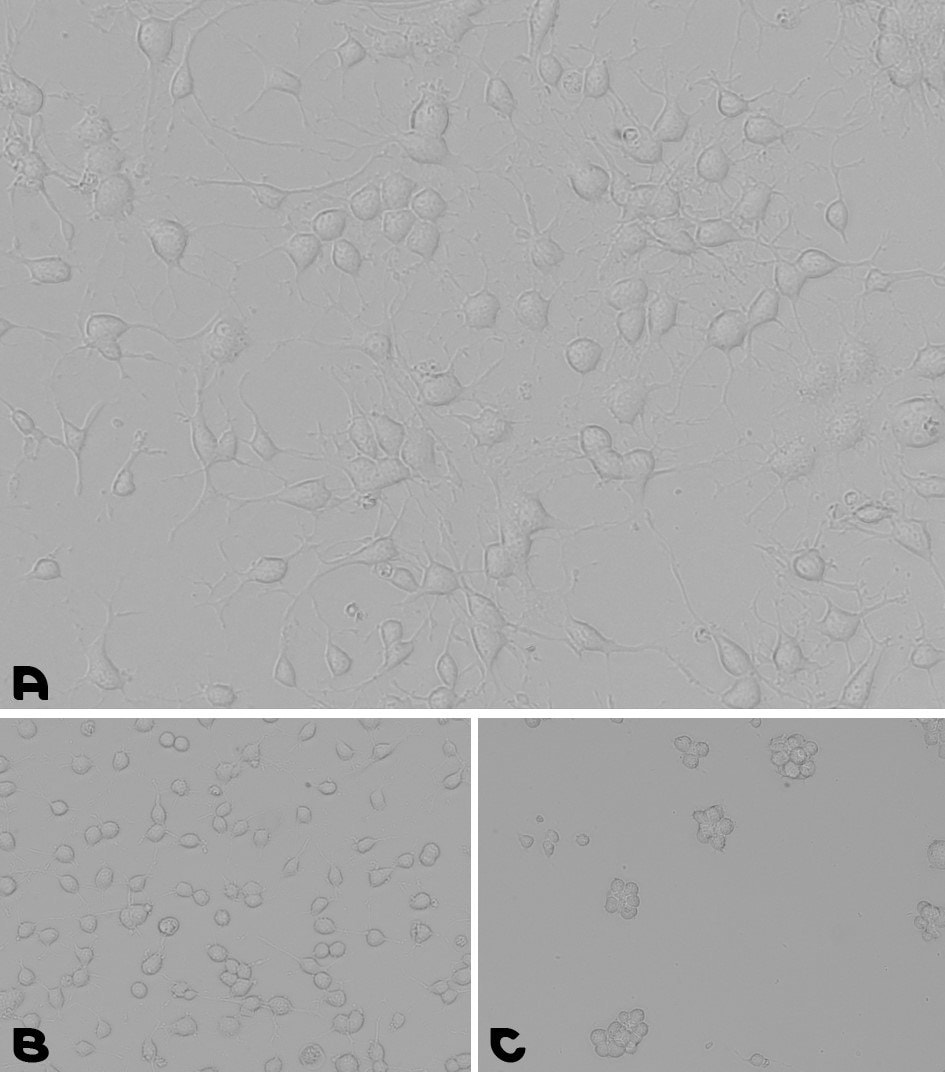
Figure 5.Neural stem cells culturing assay. A. Coating prepared by a 1-hour incubation at 37°C with the pre-mixed coating Solution of Laminin with PLL. B. Coating prepared by an overnight incubation with PLL, followed by a wash and a second overnight incubation with Laminin. C. NSCs were cultured for 48 hours on a TC plate without any coating.
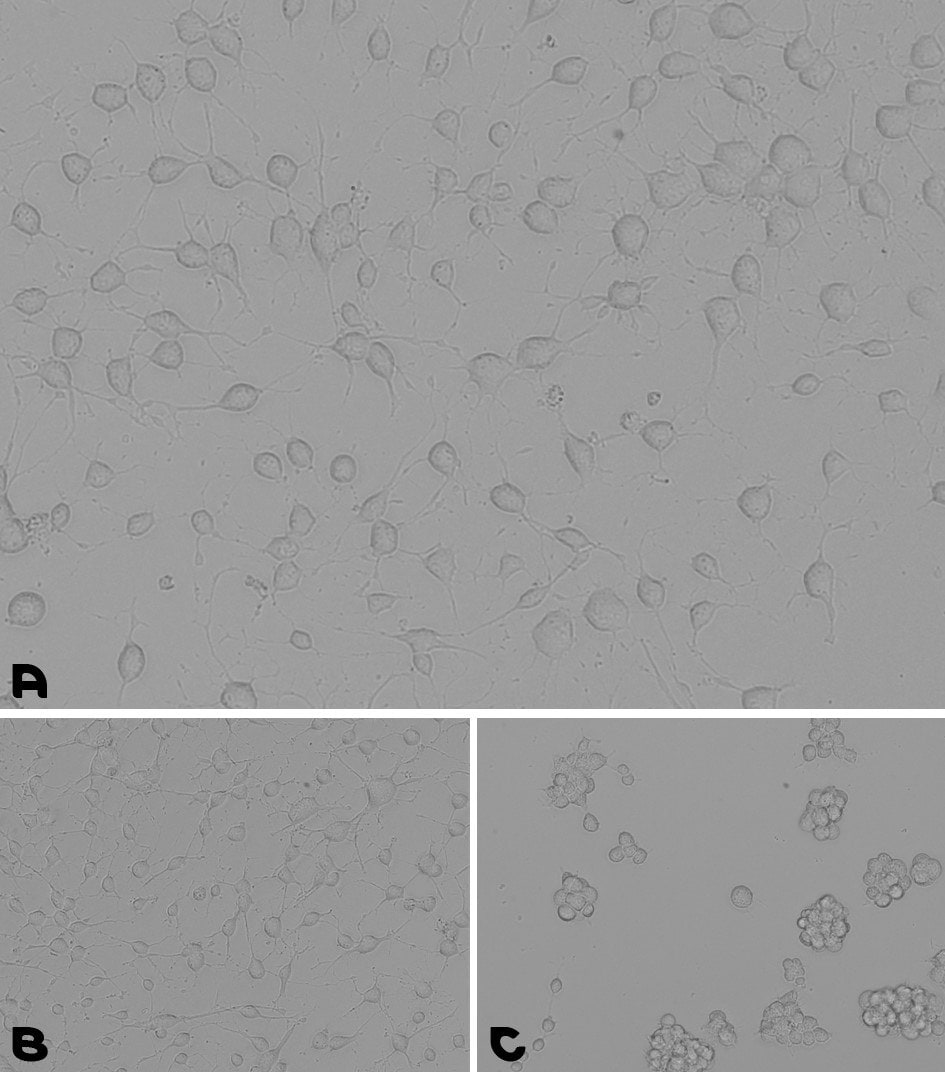
Figure 6.Neural stem cells culturing assay. A. Coating prepared by a 1-hour incubation at 37°C with the pre-mixed coating Solution of Laminin with PLO. B. Coating prepared by an overnight incubation with PLO, followed by a wash and a second overnight incubation with Laminin. C. NSCs were cultured for 48 hours on a TC plate without any coating.
Our Fibronectin - Gelatin Coating Solution mix is suitable for use with serum-free or reduced-serum cultures, and it is especially useful for culturing HL-1 cardiac muscle cell line and endothelial cells.
In Figure 7, different cell lines (BHK21 cells, CHO cells, F9 cells, and HL-1 cardiac muscle cell line) were cultured on the Fibronectin/Gelatin Coating Solution (FG001). All cell lines demonstrated outstanding attachment and proliferation, confirming the effectiveness of the ready-to-use Fibronectin - Gelatin Coating Solution.
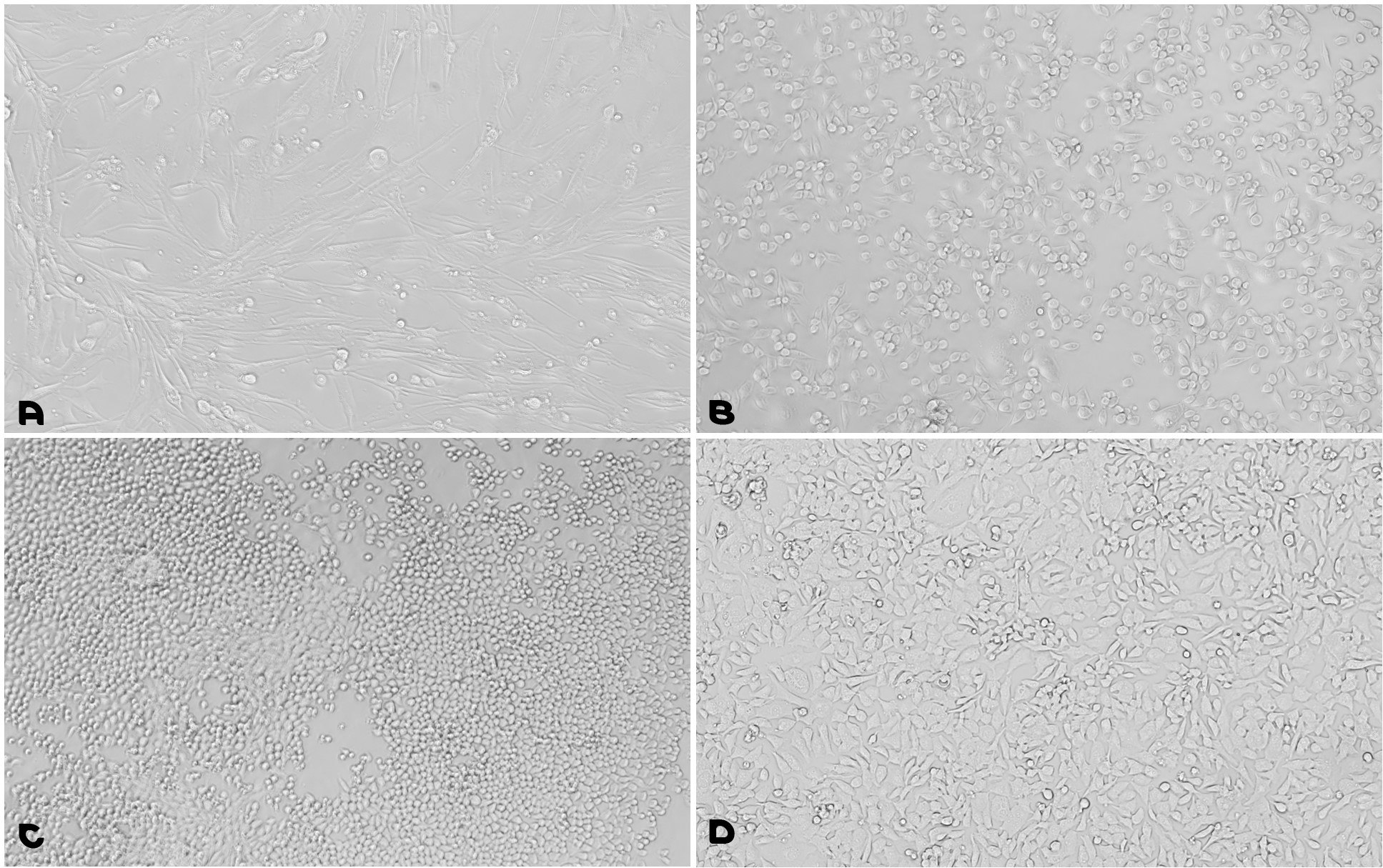
Figure 7.Cell Line Growth Using Fibronectin - Gelatin Coating Solution. A. BHK21 cells (fibroblasts from hamster kidney); B. CHO cells (Chinese Hamster Ovary); C. F9 Cells (mouse testis embryonal carcinoma); D. HL-1 cardiac muscle cell line (SCC065).
Our ready-to-use, pre-mixed attachment factor solutions offer a time-saving and efficient alternative for cell culture assays, enabling researchers to initiate their experiments on the same day as plate coating. The provided experimental results demonstrate the suitability of these mixes for various cell types, making them valuable tools for streamlined and successful cell culture experiments.
Coated Strips and Plates for Cell Adhesion
Millicoat® Cell Adhesion Strips
Millicoat® cell adhesion strips are 12 removable 8-well strips the fit within a plate frame, providing for convenience and flexibility when designing cellular assays. The wells in rows A-G are precoated with an ECM protein, such as fibronectin or vitronectin, and row H is coated with BSA to serve as a negative control. The cells can then be seeded on the coated surfaced, and adherent cells can be fixed and stained.
Cytosoft Plates
The rigidity of the substrate a cell grows on influences its cellular functions. CytoSoft® plates are coated with thin layer of biocompatible silicone, with various rigidities covering a broad physiological range. The surface of the gels forms stable covalent bonds with proteins, facilitating the coating of the gel with attachment factors (ECM components) and plating cells. CytoSoft® plates have the following attributes:
- Optically clear and have low auto-florescence
- Silicone gels are not susceptible to hydrolysis
- Silicone gels are stable, they do not dry nor swell
- Resistant to tearing or cracking
- Rigidities nearly unchanged during extending storage periods
- Trypsin and collagenase can be used to harvest cells
- Resistant to biochemical breakdown after enzyme treatment
Other ECM Proteins in Cell Culture
Coating of culture surface with ECM proteins and synthetic polymers greatly influences cell behavior. The response observed is dependent on both cell type and coating used as substrate. Cells in contact with the attachment factors survive longer and can also be grown in absence of serum factors.3 The attachment factors can sequester and store growth factors, controlling spatio-temporal regulation of factors and facilitating cross talk between growth factor receptors and ECM receptors. It also defines mechanical properties and instructs cells to differentiate under permissive conditions. ECM proteins induce intracellular signaling through cell-surface receptor in synergy with growth factor signaling.12 As cell culture is evolving, more components and combinations are needed to better mimic the in-vivo conditions of tissues and decipher the language of extracellular matrix between cells.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?