BINOL and Derivatives
BINOL and its derivatives are one of the mostly widely used classes of ligands in asymmetric synthesis; being utilized in a broad array of reactions including: Diels–Alder, carbonyl addition and reductions, Michael additions, as well as many others. While tremendous success has been obtained with the BINOL platform, other C2-symmetric diol ligands have attracted considerable attention. Among these are the vaulted biaryl ligands developed by Wulff and co-workers. Both vaulted 3,3'-biphenanthrol (VAPOL) and vaulted 2,2'-binaphthol (VANOL) have proven to be excellent ligands in catalytic asymmetric Diels–Alder, imine aldol, and aziridination reactions (Figure 1)1. Recently the phosphoric acid derivative of VAPOL was shown to be effective as a chiral Brønsted acid catalyst. In many of the examples illustrated herein, the vaulted biaryls give both higher yields and higher inductions than the same reactions using a BINOL ligand.
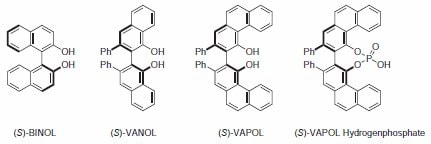
Figure 1.
Asymmetric Diels–Alder Reaction
Very early on, a catalyst generated from Et2AlCl and VAPOL was shown to be an effective catalyst for the asymmetric Diels-Alder reaction2. As shown in Scheme 1, the cycloaddition of acrolein with cyclopentadiene in the presence of the VAPOL-derived catalyst gave high conversions and excellent stereoselectivities for the exo isomer in very high optical purity. Analogous reactions with the BINOL-derived catalyst provided the cycloadduct in high yield, but in very low enantiomeric excess (13–41%).
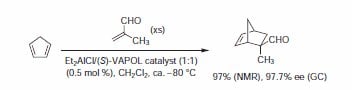
Scheme 1.
Asymmetric Aziridination Reaction
Aziridines are important building blocks in organic synthesis because they allow for convenient access to amines, amino alcohols, diamines, and other useful nitrogen-containing molecules. Most contemporary methods of chiral aziridine preparation have relied on the chiral pool. Recently, the Wulff group has developed a robust catalytic asymmetric aziridination reaction providing optically active aziridines in high yields and selectivities. The reaction relies on the addition of commercially available ethyl diazoacetate (EDA) to benzhydryl imines in the presence of arylborate catalysts prepared from vaulted aryl ligands and B(OPh)3.3The aziridination reaction exhibits excellent selectivities for the cis isomer, and high enantiomeric excesses are obtained. The resultant benzyhydrylprotected aziridines undergo a variety of reactions, including: deprotection, reductive ring opening, or alkylation reactions (Scheme 2, Table 1). The asymmetric synthesis of leukointegrin LFA-1 antagonist BIRT-377 utilized an aziridination/alkylation methodology to provide the hydantoin target in excellent overall yield.
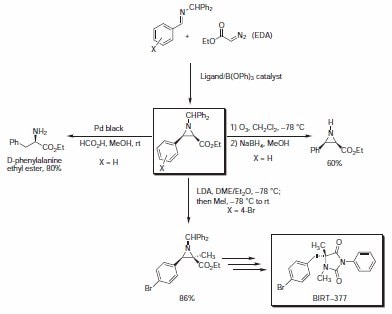
Scheme 2.
The highly expeditious synthesis of the antibacterial agent (–)-chloramphenicol utilized the asymmetric aziridination reaction, followed by a nucleophilic ring opening of the aziridine with dichloroacetic acid with a subsequent acyl group migration (Scheme 3, Table 2). Both VANOL and VAPOL gave higher yields and stereoselectivities than the BINOL-derived catalyst.
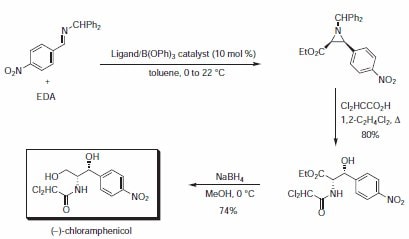
Scheme 3.
Asymmetric Aldol Reaction
Asymmetric imine aldol reactions are also catalyzed by vaulted biaryl-derived catalysts, providing an important method for the synthesis of chiral β-amino esters. The addition of silyl ketene acetals to aryl imines in the presence of either Zr-VANOL or Zr-VAPOL catalysts proceeds with high asymmetric induction and in excellent yield (Scheme 4, Table 3). Significantly, both catalysts exhibit substantially higher levels of induction over the analogous BINOL-derived catalyst4.

Scheme 4.
Chiral Brønsted Acid
Antilla and co-workers demonstrated VAPOL hydrogenphosphate to be a useful chiral Brønsted acid catalyst in the addition of sulfonamides to Boc-activated aryl imines (Scheme 5)5. The resultant N,N-aminal products were prepared in high yields with impressive enantiopurities. The identical reaction with a BINOL-derived Brønsted acid catalyst gave an excellent yield (95%), but a dismal level of asymmetric induction (<5% ee) was obtained. A variety of sulfonamides and aryl imines are active in the imine amidation reaction, and the resultant protected aminals are shelf-stable compounds.

Scheme 5.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?