CBS Catalysts
2-Methyl-CBS-oxazaborolidines for asymmetric reduction
Since 1987, the series of chiral oxazoborolidines known as CBS catalysts (after the work of Corey, Bakshi, and Shibata) have been used for catalytic reduction of prochiral ketones1-2, imines3-5, and oximes6 to produce chiral alcohols, amines, and amino alcohols in excellent yields and ee’s. We are pleased to offer both enatiomers of 2-methyl-CBS-oxazaborolidine as a dry reagent, in addition to our current offerings as a 1M solution in toluene.
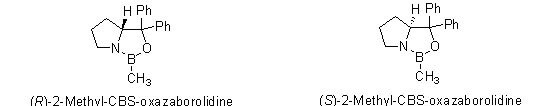
o-Tolyl-CBS-oxazaborolidines for asymmetric synthesis
We are also pleased to offer the o-tolyl-CBS-oxazaborolidine as a 0.5M solution in toluene for your research needs. When protonated with trifluoromethanesulfonimide (Product No. 46463-5), these chiral oxazaborolidines generate chiral Lewis acids which have demonstrated great utility in the enantioselective Diels-Alder reaction (Scheme 1).7
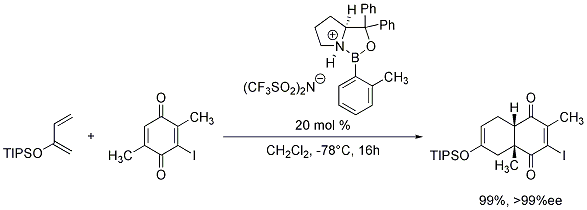
Scheme 1.o tolyl cbs oxazaborolidine
References
To continue reading please sign in or create an account.
Don't Have An Account?