Fmoc Resin Cleavage and Deprotection
Table of Contents
Introduction
Having successfully synthesized a protected peptide, one is confronted with a difficult task of having to simultaneously detach the peptide from the resin support and remove all the side-chain protecting groups of the amino acid residues to yield the desired peptide. In Fmoc SPPS, this step is normally carried out by treating the peptidyl resin with TFA. During this process, highly reactive cationic species are generated from the protecting groups and the handles on the resin, and these can, unless trapped, react with, and hence modify, those residues which contain nucleophilic functional groups: Trp, Met, Tyr, and Cys. To prevent this, various nucleophilic reagents (known as scavengers) are added to the TFA to quench these ions1, 2
Many universal cleavage mixtures have been advocated, the most popular of which is Reagent K (TFA/water/phenol/thioanisole/EDT [82.5:5:5:5:2.5])1. However, advances in protecting group and linker technology, particularly the introduction of Fmoc-Trp(Boc) and Fmoc-Arg(Pmc/Pbf) derivatives, such complex mixtures containing toxic and malodorous reagents are no longer necessary, except in exceptional circumstances.
Most problems can be ameliorated by the appropriate choice of protected amino acid derivative and resin (Table 1). If these recommendations are followed, the use of TFA/TIS/water (95:2.5:2.5) will suffice for most sequences. There are, of course, sequences, especially those which contain cysteine and numerous t-butyl protected residues, for which this mixture does not give satisfactory results; in these cases, the addition of EDT to the mixture or the use of reagent K is recommended. Nevertheless, as a general, non-malodorous cleavage cocktail, this mixture has proved remarkably effective.
For those who do not wish to use the recommendations given in Table 1, the flow-chart shown in Figure 1 will aid in the selection of the most appropriate mixture.
Removal of N-terminal Fmoc group
Before acid cleavage of the peptidyl resin can be performed, the N-terminal Fmoc group must be removed using piperidine. Check with the instruction manual of your synthesizer; many synthesizers will automatically program the removal of the N-terminal Fmoc-group as a last step in the synthesis.
Preparing peptide resin for cleavage
The peptide resin should be thoroughly washed, especially when DMF is used during synthesis as it is nonvolatile, and residual basic DMF can have a marked inhibitory effect on TFA-acidolysis. For PEG and polyacrylamide-based supports, washing with a mildly acidic reagent, such as acetic acid which does not cause the release of the peptide, is desirable since these types of resin tend to hold onto DMF3. Thorough washing and drying must be carried out before cleavage (Method 1).
Note: Acetic acid should not be used for washing of extremely acid-labile Rink acid, TGT, or 2-chlorotrityl resins.
Method 1: Preparing peptide resin for cleavage
- Place the peptide resin in a sintered glass funnel and apply some suction.
- Wash with DMF, acetic acid, then with DCM several times. Wash further with MeOH (polystyrene) or ether (polyacrylamide) to shrink the resin.
- Remove the peptide resin and dry under a high vacuum for 4 h, or preferably o/n, over KOH.
TFA cleavage and deprotection
Optimum cleavage conditions are very much dependent on the individual amino acid residues present, their number and sequence, the side-chain protecting groups, and the type of linker attached to the resin.
Due to the variability in the behavior of different peptidyl resins, it is recommended that a preliminary small scale cleavage of peptide resin using 20-50 mg sample is carried out to determine the optimum cleavage conditions, such as the choice of scavenger(s) and length of reaction.
This will enable the extent of cleavage (e.g. by quantitative analysis of the reference amino acid attached to the linker, where appropriate) and the quality of the crude cleaved peptide (by HPLC and amino acid analysis) to be determined. For the majority of peptides, provided the recommendations given in Table 1 are followed, cleavage can be affected with TFA/TIS/water (95:2.5:2.5). In cases where problems do occur, the use of Reagent K, or the addition of EDT to the above mixture, will generally provide a satisfactory solution.
In the case of the Rink Amide resin, the phenyl benzyl ether bond, which links the handle to the resin, is acid sensitive and can be broken, especially when product release is sluggish during the cleavage reaction, resulting in colored by-products which are not easily removed from the product by simple washes. This can be avoided using the 2-step procedure outlined in Method 3, or better by using silane scavengers. These steps are not necessary with resins incorporating the more stable modified Rink linkers, such as the Rink Amide AM, Rink Amide MBHA resin, and NovaSyn® TGR resins.
Methionine, cysteine, and tryptophan are extremely susceptible to alkylation by cations produced during the cleavage process. The reaction of tryptophan, methionine, or cysteine with t-butyl cations results in modification of the product peptide; reaction with the linker cation gives irreversible reattachment of the peptide to the resin3. With methionine, a further reaction can occur giving rise to homoserine and fragmentation of the peptide chain. By adding scavengers to the cleavage mixture, these side reactions can be largely suppressed. One exception is sulfonation of tryptophan by the products formed on the cleavage of Mtr, Pmc and Pbf protected arginine residues4. Fortunately, this side reaction can be eliminated by using Fmoc-Trp(Boc)5-8. This derivative also suppresses the reattachment of C-terminal Trp residues to the cation generated at the resin linker. Sulfonyl-based protecting groups have also been shown to be associated with the formation of N-sulfonated Arg9 and O-sulfonated Ser and Thr10.
The most commonly used scavenger is EDT. Not only is it an extremely good scavenger for t-butyl cations, but it assists in the removal of the trityl protecting group from cysteine and is particularly effective in preventing acid-catalyzed oxidation of tryptophan residues.
Suppression of acid-catalyzed Met oxidation can be carried out by including ethyl methyl sulfide (EMS), EDT, or thioanisole into the scavenger mixture; although methionine sulfoxide formation can be minimized by carrying out the cleavage reaction under nitrogen, ensuring only peroxide-free ether is used for product precipitation, and that all solvents are thoroughly degassed before use. Thioanisole is also known to accelerate Arg(Mtr/Pmc/Pbf) removal in TFA; however, it is advisable to exercise care when using this reagent as there is evidence to suggest that it can cause partial removal of Acm, tButhio, or tBu protecting groups from Cys residues11.
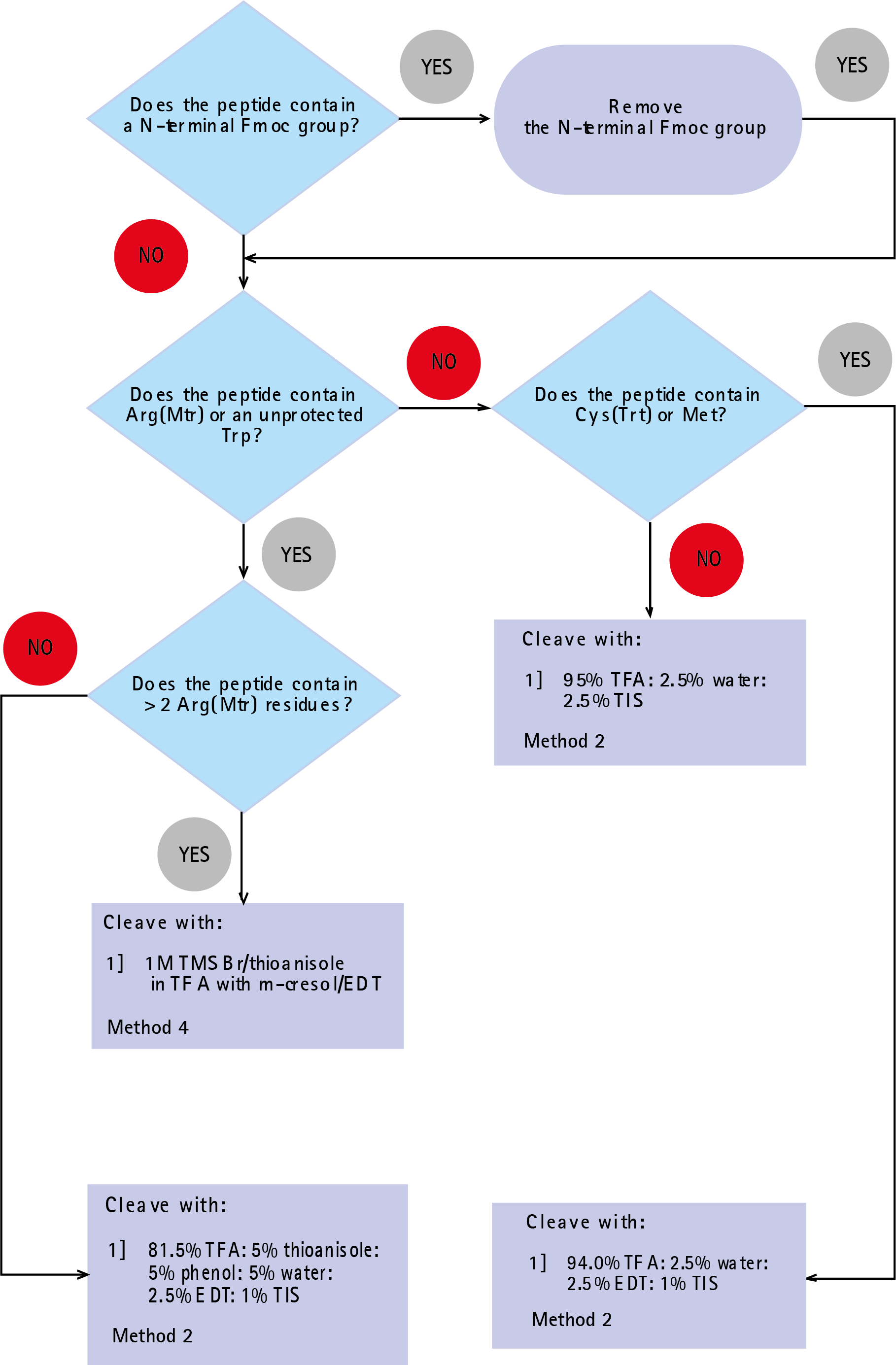
Figure 1: Flow-chart for selecting cleavage cocktail for Fmoc SPPS
Phenol is thought to offer some protection to Tyr and Trp residues1. Trialkylsilanes, such as TIS and TES, has been shown to be effective, non-odorous substitutes for EDT12, particularly for peptides containing Arg(Pmc) and Trp(Boc)5, 8. These reagents are also very efficient at quenching highly stabilized cations liberated on the cleavage of Trt 12, Tmob13, and the Rink Amide linker, and therefore their use is strongly recommended when these moieties are present.
When several of the less acid-labile protecting groups are present in a peptide or the peptide is long and therefore contains numerous protecting groups, cleavage time usually needs to be extended significantly. The Mtr group is less acid-labile than PMC or Pbf groups, and its complete removal can take as long as 24 hours. In such cases where Trp is present with several Mtr protecting groups, it is extremely useful to be able to optimize the cleavage conditions by monitoring the removal of this protecting group by HPLC. A compromise needs to be made between partially tryptophan-modified peptide and incomplete deprotection of Arg(Mtr). Therefore, with peptides containing Trp, the use of Trp(Boc)-derivatives is strongly recommended to avoid modification of the tryptophan side chain.
With long peptides, it can be necessary to use an extended cleavage time to completely remove all side-chain protection. If complete deprotection is not achieved in 6 hours, the peptide should be precipitated with ether, and the cleavage repeated with fresh reagents. Test cleavages should be performed to find the optimum cleavage regime. Incomplete side-chain deprotection is often overlooked as the cause for failure in the synthesis of long peptides.
Problems have been observed with sluggish deprotection of N-terminal Asn(Trt) residues. These can easily be overcome by extending the cleavage time to 4 hours or using Asn(Dmcp) in place of Asn(Trt).
Method 2: General TFA cleavage
CAUTION: TFA is an extremely corrosive liquid; great care must be taken when using this reagent. Proper eye protection, lab coat, and gloves are mandatory. Follow local, state/provincial, and federal safety regulations. Use in an efficient fume hood.
- Place dry resin in a flask and add a TFA solution containing appropriate scavengers (10-25 ml/g resin, Figure 1). NOTE: a calculation should be made to ensure sufficient scavenger is present for the quality of peptide and protecting groups present. Stopper the flask and leave to stand at rt with occasional swirling. Reaction time depends upon the sequence (see “Monitoring the cleavage reaction”, below).
- Remove the resin by filtration under reduced pressure. Wash the resin twice with TFA. Combine filtrates, and add (drop-wise) an 8-10 fold volume of cold ether. Sometimes it is necessary to evaporate most of the TFA to achieve good precipitation of the crude peptide. The ether can be cooled with ice to further assist precipitation.
- Isolate the peptide as described in Method 17.
Method 3: Two-stage procedure for detachment/deprotection of Rink amide resin
- Slurry the resin in 10% TFA in DCM and pour it into a glass funnel with a fine sinter.
- Allow the solvent to percolate slowly through the resin bed. Wash the resin with 5% TFA, allowing it to pass through the resin bed slowly. The detachment is an acid-catalyzed equilibrium, so it is important to continually remove the detached peptide by using this flow method. Carrying out the reaction in a flask will not achieve complete detachment. Yields can be improved by the addition of 1-5% TIS to the cleavage mixture.
- Remove the excess TFA/DCM under reduced pressure and complete the deprotection with 95% TFA plus scavengers, according to the amino acid composition (see Figure 1).
Monitoring the cleavage reaction
The presence of Mtr protected arginine in a peptide necessitates protracted reaction times varying from 3 to 6 hours depending upon the choice of scavengers used (Figure 1). Multiple arginine residues can require an extension of reaction times up to 24 hours. In such cases, it is extremely useful to be able to optimize the cleavage conditions by monitoring the removal of this protecting group by HPLC.
General protocols involving strong acids
As an alternative to TFA, for rapid deprotection of less acid-labile side-chain protecting groups such as Arg(Mtr/Pmc/Pbf), Asn(Mbh), and Gln(Mbh), stronger acids can be used with appropriate scavengers with no report of side reactions.
Resin cleavage using trimethylsilyl bromide14
The long cleavage times often found necessary for the complete deprotection of peptide-containing multiple Arg(Mtr) residues can lead to serious degradation in product quality. In particular, prolonged exposure of tryptophan-containing peptides to EDT in TFA can lead to modification of tryptophan residues due to dithioketal formation. Cleavage with trimethylsilyl bromide (TMSBr) eliminates these problems since this reagent has been shown to cleanly deprotect up to 4 Arg(Mtr) residues in 15 minutes. Furthermore, this method has been found to completely suppress the formation of sulfonation by-products, even when unprotected tryptophan is used15.
- Add TMSBr (1.32 ml) to a solution of EDT (0.50 ml), m-cresol (0.1 ml) and thioanisole (1.17 ml) in TFA (7.5 ml) cooled to 0°C. Add the peptide resin (200 mg) and allow the mixture to stand for 15 min under a blanket of N2 at 0°C.
- Remove the resin by filtration under reduced pressure. Wash the resin twice with a clean TFA. Combine filtrates, and add (drop-wise) an 8-10 fold volume of cold ether. Sometimes it is necessary to evaporate most of the TFA to achieve good precipitation of the crude peptide. The ether can be cooled with ice to further assist precipitation.
- Isolate the peptide as described in Method 17.
NOTE: Occasionally an additional treatment of the peptide with ammonium fluoride is required to reverse any silylation which may have occurred.
Cleavage from very acid-sensitive resins
The Rink Acid resin16, 2-chlorotrityl17, HMPB18, NovaSyn TGT19, and Sieber resins20 contain highly acid-sensitive linkers and are suitable for the synthesis of protected peptides.
HMPB resins & Sieber amide resins
Fully protected peptide acids can be generated from the HMPB linker21 and protected amides from the Sieber amide resin20. However, careful experimentation is essential if a premature loss of side-chain protecting groups is to be avoided. Repetitive treatment of the peptidyl resin with a solution of a 1% solution of TFA in dichloromethane in tandem with minimum reaction times will give the best results.
Ideally, the cleavage should be carried out in a sealable sintered glass funnel to prevent evaporation of the highly volatile DCM, and the filtration should be carried out by applying nitrogen pressure rather than using a vacuum.
If the peptide contains Met or Trp, 1% EDT should be added to the cleavage mixture to prevent reattachment of the peptide. If the peptide contains a C-terminal Trp residue, the use of Trp(Boc) is strongly recommended21.
In this batch-wise procedure, the acid strength increases step-wise, as determined by the amount of TFA-buffering groups present. The maximal concentration of peptide may be contained in the first or in one of the later washes, depending on the buffering capacity of the amide bonds and other functional groups present. The side-chain protecting groups of the t-butyl type as well as Trt (on Asn and Gln) remain completely intact during this process21.
Method 5: Cleavage with dilute TFA
- Pre-swell the dry resin (1 g) with DCM in a sealable sintered glass funnel and remove excess DCM.
- Add 1% TFA in dry DCM (10 ml), seal funnel, and shake for 2 min. Filter solution by applying nitrogen pressure into a flask containing 10% pyridine in methanol (2 ml).
- Repeat step 2 up to 10 times, wash the residual protected peptide from the resin with 3 x 30 ml DCM, 3 x 30 ml MeOH, 2 x 30 ml DCM, 3 x 30 ml MeOH, and check filtrates by TLC or HPLC.
- Combine filtrates which contain product and evaporate under reduced pressure to 5% of the volume. Add water (40 ml) to the residue and cool mixture with ice to aid precipitation of the product.
- Isolate product by filtration through a sintered glass funnel. Wash product three times with fresh water. Dry sample in a desiccator under vacuum over KOH, and later over P2O5.
2-Chlorotrityl, NovaSyn® TGT, and NovaPEG Trt resins
2-Chlorotrityl17, NovaSyn® TGT19, and NovaPEG Trt resins can be cleaved with 1% TFA, as described above, or under milder conditions with AcOH or TFE 22 to produce protected peptides. When preparing protected peptides, the use of Fmoc-His(Clt)-OH is particularly recommended for the introduction of histidine. This helps avoids partial side-chain deprotection of histidine which can occur when His(Trt) is used.
Method 6: Cleavage with TFE/DCM
1. Treat the peptidyl resin at rt with TFE/DCM (2:8) for 3 x1 h.
2. After the appropriate time, remove resin by filtration, wash 3 times with cleavage mixture.
3. Evaporate the solution to dryness and precipitate protected peptide with ether.
The detached, fully protected peptide will be very hydrophobic and may require to be extracted further from the resin with DMF, DMSO. The completeness of the cleavage can be checked by the TLC of the filtrates. Purify by low-pressure chromatography on silica gel, HPLC on phenyl silica, or by recrystallization.
Peptides attached by the HMBA and oxime linker
For the synthesis of peptide carboxamides, the HMBA linker has been largely superseded by those of the Rink Amide type. Nevertheless, this linker is still one of the most flexible of the peptide-resin linkers. Peptides can be released from linker with many different nucleophiles to yield peptides with a variety of functional groups at the C-terminus3, 22 -25 (Table 2). These protocols are also compatible with the oxime linker used in Boc SPPS.
Method 7: Methanolic ammonia cleavage to give peptide amides3, 23
- Place dry resin in a flask and add 95% aqueous TFA (20 ml/g). Stopper the flask and leave to stand at rt for 1 h with occasional swirling.
- Isolate the resin by filtration under reduced pressure and wash with TFA. Discard the filtrate. Wash the resin with DCM, 10% DIPEA in DCM, DCM. Dry the resin under vacuum over P2O5 o/n.
- Place the dried resin in a clean, dry pressure vessel and sufficient DMF to swell the resin, followed by a solution of dry methanol saturated with ammonia at 0°C (20 ml/g).
- Seal the flask and let it warm to rt. Leave to stand for 18 h and then cool the flask to 0°C again. Carefully open the cooled vessel and filter the resin through a sintered glass funnel.
- Wash the resin first with methanol, and then with TFA into a separate flask to remove any methanol insoluble peptide.
- Evaporate the filtrates separately to dryness on a rotary evaporator. Precipitate peptide with ether and isolate it by filtration.
NOTE: If the peptide resin is not thoroughly dried prior to this cleavage procedure, peptide acid may be obtained as a by-product.
Method 8: Cleavage with hydrazine to give the C-terminal hydrazide24
If required, the side-chain protecting groups should be first removed following Method 7, steps 1 & 2.
- Suspend the peptide resin in DMF and add a solution of 5% hydrazine hydrate in DMF (20 ml/g). Leave to stand for 1 h at rt.
- Filter the resin through a sintered glass funnel and wash the resin first with DMF, and then with TFA into a separate flask to remove any DMF insoluble peptide.
- Evaporate the filtrates separately to dryness on a rotary evaporator. Precipitate peptide with ether and isolate it by filtration.
Method 9: Cleavage with alkali to give the free acid3
The side-chain protecting groups should be first removed using Method 7 steps 1 & 2.
- Pre-swell the resin in dioxane.
- Cool 0.1 M NaOH/ dioxane (1:3, 20 ml/g) to 0°C in an ice/water bath. Add the peptide resin and leave to stand for 15 min at rt.
- Filter the resin using a glass sintered funnel into a flask containing 0.1 M HCl (5 ml/g). This flask should be cooled in an ice bath to prevent warming as the base solution is neutralized.
- Wash the resin with water and adjust the pH of the filtrate to 7.0. Lyophilize the filtrate and remove the sodium chloride by gel-filtration.
Method 10: Cleavage with methanol/DIPEA to give the methyl ester24
The side-chain protecting groups should be first removed using Method 7, steps 1 & 2.
- Place the resin in a clean flask and add sufficient DMF to swell the resin. Cleave o/n with DIPEA/MeOH/DMF (1:5:5, 50 ml/g).
- Wash the resin first with MeOH/DMF and then with TFA into a separate flask to remove any methanol insoluble peptide.
- Evaporate the filtrates separately to dryness on a rotary evaporator. Precipitate peptide with ether and isolate it by filtration.
If the yields are low, repeat the reaction with fresh reagents at 50°C.
Method 11: Cleavage with borohydride to give the peptide alcohol25
This method is only applicable to TG and PEGA-type resins.
The side-chain protecting groups should be first removed using Method 7, steps 1 & 2.
- Treat the peptidyl resin (1.0 g) with 50% aq. EtOH, and allow to drain. Add NaBH4 (126 mg) in 3 ml 50% aq. EtOH and gently agitate for 4 h.
- Remove the resin by filtration and wash with 50% aq. EtOH (40 ml) to give a solution of the peptide at pH~9.
Before cleaving the peptide from HMBA derivatized resins, it is important that the side-chain protecting groups are removed, especially if the peptide contains Asp or Glu. This is achieved by treating the peptidyl resin with 95% aq. TFA. If the peptide contains Arg(Mtr), the Arg residue is best deprotected after cleavage of the peptide from the resin in an additional step by treatment with reagent K.
Peptides attached by the sulfamylbutyryl resins, to produce thioesters
Displacement of a peptide fragment with a thiol from an alkylated sulfamylbutyryl resin was first described by Pessi and coworkers26 and then later by others27 – 29 (Figure 2). Following chain assembly, the resin is activated by alkylation of the sulfonamide nitrogen, usual treatment with iodoacetonitrile, or TMS-CHN2. Activation with iodoacetonitrile produces a more reactive intermediate, whereas with TMS-CHN2 the actual process of activation is more efficient. Activation methods have been reviewed in ref.30. The resulting N-alkyl-N-acylsulfonamide is then cleaved by treatment with either benzylmercaptan26 or ethyl mercaptopropionate /thiophenol27. However, the combination of activation with TMS-CHN2 and displacement with ethyl mercaptopropionate/thiophenol appears to be optimal
(Method 12). The use of 2M LiBr in THF as the cleavage solvent has been shown to lead to greatly improved yields of peptide thioester31.
The resulting protected peptide thioester is then treated with TFA and the appropriate scavengers to give the deprotected peptide ready for ligation.
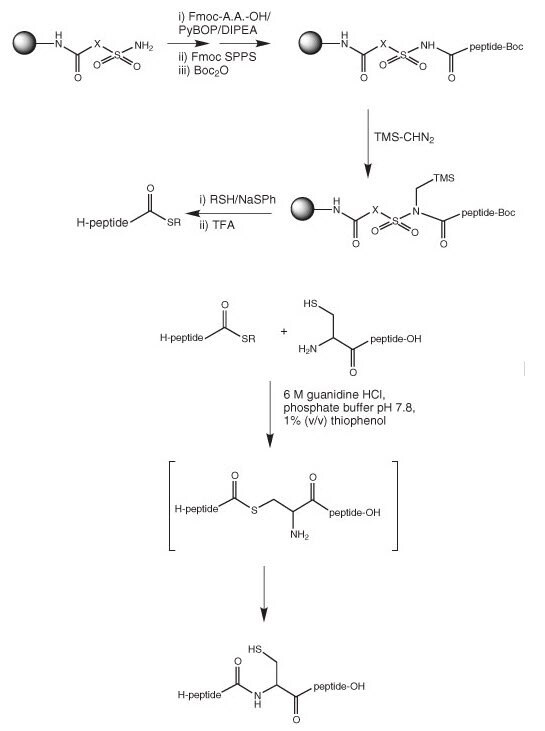
Figure 2:Synthesis of thioesters using sulfamylbutyryl resins
Method 12: Thioester ligation with sulfamyl resins
Activation of acylsulfamyl resins
- Pre-swell the resin (0.1 mmole) in dry THF in a 10 ml polypropylene syringe fitted with a 20 mm polyethylene filter.
- Add 5 ml of 1 M TMS-CHN2 in dry hexane/THF (1:1). Cap the syringe.
- Agitate gently for 2 h. Wash resin with THF and use immediately, or wash with THF, then DCM and dry in vacuo.
Cleavage of thioester
- Pre-swell methylated resin in DMF for 1 h before use.
- Add ethyl-3-mercaptopropionate (50 eq.) and sodium thiophenoxide (0.5 eq.), cap the syringe and agitate the mixture gently for 24 h.
- Remove the resin by filtration and wash it three times with DMF.
- Combine the filtrates and evaporate to dryness on a rotary evaporator. Triturate the product with ether.
- Treat the residue with TFA/water/TIS /phenol (88:5:2:5) for 2 h at rt.
- Add the cleavage solution drop-wise to 10 volumes of ether, and isolate the product by filtration or centrifugation using standard methods. Purify product by RP-HPLC.
Ligation of unprotected peptide fragments
- Dissolve peptide thioester (1 eq.) and N-terminal-Cys peptide (1 eq.) in a screw-cap tube containing degassed 0.1 M sodium phosphate buffer, pH 7.8. If necessary, the solution can also contain guanidine hydrochloride (up to 6 M) to add dissolution of the peptide components. The final concentration of the peptides should be 2-5 mM.
- Add thiophenol (1% by volume of total solution), flush with nitrogen, recap tube, and agitate mixture vigorously. The progress of the reaction can be monitored by HPLC. The reaction is typically over in 5-16 h.
- Acidify the reaction with TFA (0.1% by volume of solution), lyophilize and purify by standard procedures.
Peptides attached by the hydrazinobenzoyl resins
Aryl diazenes, produced by oxidation of aryl hydrazines, readily decompose under mild conditions to give arenes and nitrogen32. This process has been exploited by Millington, et al.33 as the basis of a novel safety-catch linker, N-Fmoc-4-hydrazinobenzoic acid (Figure 3), which is supplied attached to NovaGel™ resin.
Method 13: Oxidative cleavage of hydrazinobenzoyl resins
To give amides using Cu(II) oxidation33
- Suspend the resin in neat amine
- Add Cu(OAc)2 (0.5 eq.) and bubble air vigorously through the resin for 4 h.
- Remove resin by filtration and wash it with DCM.
- Evaporate combined organic filtrates to dryness. Redissolve in DCM and wash with 1 M KHSO4, water, and sat. NaCl.
- Dry the organic layer over Na2SO4 and evaporate to dryness.
To give ester or amine using NBS34
- Suspend the resin in dry DCM
- Add NBS (2 eq.) and dry pyridine (2 eq.). Agitate gently for 5 min.
- Remove resin by filtration and wash it with dry DCM and dry THF.
- Resuspend resin in dry DCM and add alcohol or amine (5 eq.) and gently agitate for 4 h.
- Remove resin by filtration and wash with DCM.
- Evaporate combined filtrates to dryness.
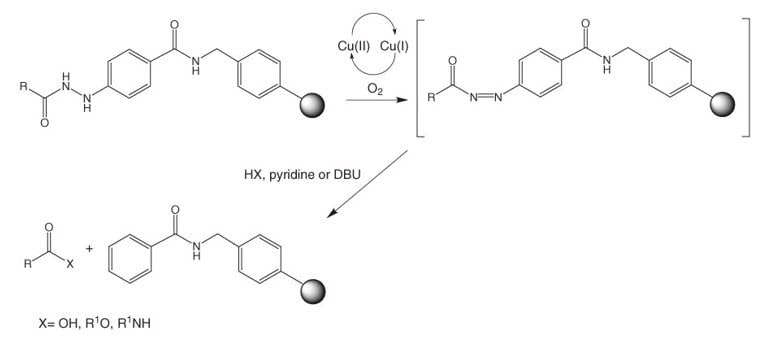
Figure 3:Applications of Fmoc-4-hydrazinobenzoyl resins.
Following removal of the Fmoc group, the resin-bound hydrazino group can be readily acylated using standard coupling methods. The support is stable to piperidine and TFA, facilitating the synthesis of both protected and unprotected peptide fragments.
Cleavage can be carried out under mild oxidative conditions by treatment with air and an appropriate nucleophile, in the presence of copper (II) acetate and pyridine (or DBU), to provide products containing a range of carboxy modifications such as acids, esters and amides. In cases where the nucleophilic component poorly solvates the resin, a co-solvent such as THF or DMF should be included in the reaction mixture.
Alternatively, the reaction can be carried out in two stages by first generating the diazene by oxidation with NBS followed by cleavage with nucleophile once excess oxidant is removed. This method has the advantage of avoiding copper contamination of the product. In the synthesis of esters of primary and secondary alcohols, Peters & Waldmann34 found NBS activation to give the highest yield. Camarero and coworkers also used this method to prepare peptide p-nitroanilides35 and peptide thioesters 36 by employing p-nitroaniline and amino acid thioesters as nucleophiles.
This resin has also been used to prepare cyclic peptides via a cyclative cleavage strategy involving intramolecular attack of the N-terminal amino group on the diazene37.
The use of Fmoc-hydrazinobenzoyl resins has recently been reviewed.38
Peptides attached to Dbz resin
Dbz resins provide a convenient approach to peptide thioesters and thioester surrogates by Fmoc SPPS39, 40 (see Figure 4).
Prior to cleavage the Dbz moiety requires conversion to the activated Nbz according to Method 14. The reaction is usually quantitative. With high loaded resins like Dawson Dbz AM resins, some cross-linking of Dbz moieties can occur. In our experience, much cleaner results are obtained with low-loaded resins like Dawson Dbz NovaSyn® TGR resin. Treatment of the Nbz resin with TFA releases the fully deprotected peptide-Nbz, which can be used directly in the NCL reaction. The Nbz peptide is obtained as a mixture of regioisomers.
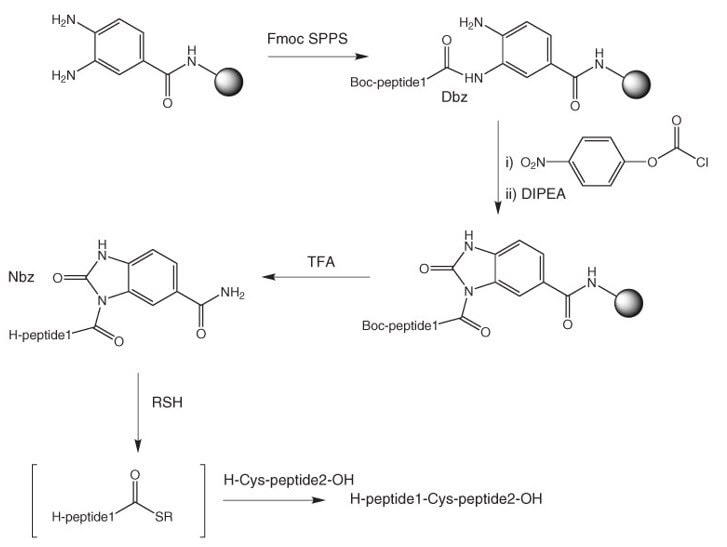
Figure 4:Synthesis of peptide thioesters using Dbz resins.
Method 14: Thioester ligation with Dawson Dbz AM resin
Synthesis & Activation
- Extend peptide chain using HBTU/HOBt/DIPEA activation, except for Gly which should be introduced using Fmoc-Gly-OPfp/HOBt. The N-terminal residue must be introduced using a Boc-amino acid. Wash the resin with DMF and DCM.
- Add p-nitrophenyl chloroformate (0.5 mmole) in DCM and leave to gently agitate under N2 for 1 h. Wash resin with DCM and add 0.5 M DIPEA in DMF (10 ml) and leave for 30 min. Wash resin with DMF and DCM.
- Cleave peptide with TFA/water/TIS 95:2.5:2.5 for 3 h.
Ligation of unprotected peptide fragments
- Dissolve purified peptide-Nbz (1 eq.) and N-terminal-Cys peptide (1.5 eq.) in a screw-cap tube containing degassed ligation buffer (0.2 M phosphate buffer, 6 M guanidine hydrochloride, 0.2 M 4-mercaptophenylacetic acid, 0.02M TCEP, pH 7.0). The final concentration of the peptides should be approximately 2 mM.
- Monitor the progress of the reaction by HPLC.
- Acidify the reaction with TFA (0.1% by volume of solution), lyophilize and purify by standard procedures.
Peptide aldehydes
There are three principle approaches for their preparation: firstly, oxidation of an appropriate peptide alcohol41; secondly, reduction of a peptide carboxylic acid derivative, such as a Weinreb ester42, 43 (see Method 15); finally, step-wise or fragment synthesis using a masked pre-formed aldehyde44 (see Method 16).
Peptides attached to Weinreb resin
The reduction of N-methoxy-N-methylamides (Weinreb amides) with LiAlH4 or DIBAL is a well-established strategy to produce peptide and a-amino-aldehydes42. Fehrentz and co-workers43 have adapted this methodology for use in SPOS, through the simple device of replacing the N-methyl group of a Weinreb amide with a linker that enables the methoxyamine to be attached to a solid support.
Due to the hindered nature of the resin-bound secondary amine, HOAt/DIPCDI or HATU/DIPEA should be used for the addition of the first residue (Method 4). The resulting support is stable to the conditions of Fmoc and Boc SPPS. After reduction with LiAlH4, Fehrentz, et al. reported obtaining peptide aldehydes in yields of 30-40%.
Method 15: Reductive cleavage of Weinreb amides
To prevent side-reactions with certain amino acid side-chain functionalities, it is normally advisable to leave all protecting groups in place; the exception to this is when the peptide contains Asp and Glu since even the t-Bu esters of these residues are reduced by LiAlH4. With peptides, the N-terminal amino group should be blocked with a Boc group; the Fmoc group is not stable under these conditions.
- Pre-swell resin (0.1 mmole) in dry THF for 1 h before use, in a round-bottomed flask equipped with a magnetic stirrer.
- Flush the flask with argon, then seal and place in an ice bath.
- Add 1 M LiAlH4 in THF (0.5 ml, 0.5 mmolea) using a syringe. Stir gently for 40 min.
- Dilute the mixture with THF and add saturated KHSO4 (0.5 ml) and K, Na tartrate (0.3 ml). Gently agitate mixture for 30 min and allow to warm to rt.
- Remove resin by filtration and wash it with DCM.
- Dry combined organic filtrates with anhydrous MgSO4 and evaporate to dryness.
aFor long peptides the quantity of LiAlH4 may need to be increased; conversely, for small organic molecules this amount may be considerably reduced.
Method 16: Cleavage of H-Thr-Gly-NovaSyn® TG resin
Cleavage from the resin and side-chain deprotection is carried out in two stages.
- Remove side-chain protecting groups with anhydrous TFA.
- Cleave the peptide aldehyde from the resin with AcOH/water/DCM/MeOH (10:5:63:21) (3 x 30 min).
The Novabiochem® product line presently offers H-Thr-Gly-NovaSyn® TG resin pre-loaded with aldehydes of Arg, Asp, Leu, Phe, and Val.
Post-cleavage work-up
DO NOT DISCARD resin support or ether until peptide analysis is complete. Both can be stored under nitrogen or argon at 4°C to prevent oxidation.
Ether precipitation
Most cleavage protocols involve precipitation of the crude cleaved peptide using cold ethyl ether. The following are general procedures for post cleavage work-up.
Method 17: Post-cleavage work-up
Peptide isolation and work-up can be achieved by either precipitation (1) or centrifugation (2). For water soluble peptides, the method in steps 3-6 can be used.
- Precipitation: Filter the precipitated peptide through hardened filter paper in a Hirsch funnel under a light vacuum. Wash the precipitate further with cold ether, dissolve the peptide in a suitable aqueous buffer and lyophilize.
- Centrifugation: Add a small volume of t-butyl methyl ether to the residue and triturate thoroughly until a free suspension is obtained. Transfer the suspension to a clean centrifuge tube, seal, and centrifuge. It is essential that a spark-free centrifuge is used for this process. Carefully decant the ether from the tube. Repeat the ether wash as necessary. Dissolve the residual solid in a suitable aqueous buffer and lyophilize.
- Water-soluble peptides: After precipitation, add water to the residue and transfer the mixture to a separating funnel. A little AcOH may be necessary to aid dissolution.
- Shake the stoppered funnel well. Release the stopper and allow the two layers to separate by standing. Isolate the lower (aqueous) layer.
- Add more water to the funnel and repeat step 4 three times. Remove the upper (ethereal) layer and store it in a clean flask. Return the combined aqueous extracts to the separating funnel.
- Add a small amount of fresh diethyl ether and repeat step 4 two or three times, each time removing the ethereal layer and returning the aqueous layer to the separating funnel. Collect the aqueous layer in a clean flask and lyophilize.
In the methods described above, the yield of peptide can often be increased if the TFA is first removed using a rotary evaporator (equipped with a CO2/acetone cold finger, oil pump, and acid trap) prior to the ether precipitation step. In most cases, after adding the ether, the peptide will adhere to the sides of the reaction flask, enabling the scavengers to be quickly and easily removed by repeated ether washing. Note: cleavage mixtures containing TFMSA and HBF4 should not be evaporated to dryness.
Since peptides prepared using the low-high HF cleavage method may contain water soluble sulfonium derivatives, it is advisable to remove these immediately prior to lyophilization as, under neutral or slightly basic conditions, they may cause alkylation of methionine and cysteine residues.
Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?