Peptide Synthesis
A peptide consists of two or more amino acids linked by an amide bond, to form a chain of amino acids typically 2 – 70 amino-acids long. Peptides are distinguished from proteins by not requiring to be folded for biological activity. Peptides occur endogenously as peptide hormones, such as angiotension, LHRH, enkephalin, and as toxins in plants and animals. Peptides are of great interest as lead compounds for drug discovery and as drugs in their own right. They also find applications in vaccines, biomaterials, histological probes and are used in large numbers as antigens to generate antibodies.
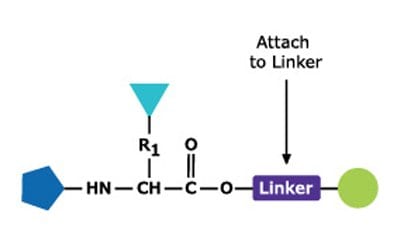
Peptides are synthesized chemically either in solution or on a solid phase. The process involves directed and selective formation of an amide bond between an N-protected amino acid and an amino acid bearing a free amino group and protected carboxylic acid. In solid phase synthesis, the carboxyl protecting group is linked to a polymer support. Following bond formation, the amino-protecting group of the dipeptide is removed, and the next N-protected amino-acid is coupled.
Featured Categories
We offer an extensive range of amino acids, resins, and reagents of unparalleled quality, including Novabiochem®, for peptide synthesis, high-throughput organic chemistry, labeling peptides, and custom manufactured products.
Our stable and tolerable NHC ligands and complexes can be used as efficient ancillary ligands to keep your work flowing in organometallic catalysis and catalysis chemistry research.
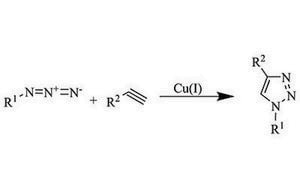
Our portfolio of click chemistry reagents includes a variety of azides, alkynes, catalysts, and ligands to accelerate your progress in chemical biology, polymer chemistry, bioconjugation, and drug discovery.
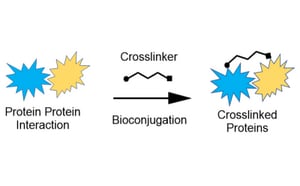
Our portfolio of linkers and crosslinkers provide structural stability and reliability in protein-protein, protein-peptide, and peptide/protein-small molecule interactions for all your bioconjugation needs.
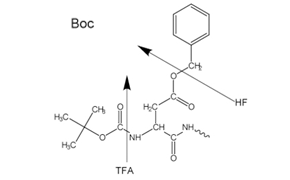
Figure 2Side-chain protecting groups for Boc solid-phase peptide synthesis (SPPS)
Solid-phase peptide synthesis (SSPS) is the most frequently used method of peptide synthesis due to its efficiency, simplicity, speed, and ease of parallelization. SPPS involves sequential addition of amino and side-chain protected amino acid residues to an amino acid or peptide attached to an insoluble polymeric support (Figure 1).
Either an acid-labile Boc group (Boc SPPS) or base-labile Fmoc-group (Fmoc SPPS) is used for N-α-protection. After removal of this protecting group, the next protected amino acid is added using either a coupling reagent or pre-activated protected amino acid derivative. The C-terminal amino acid is anchored to the resin via a linker, the nature of which determines the conditions required to release the peptide from the support after chain extension. Side-chain protecting groups are often chosen so as to be cleaved simultaneously with detachment of the peptide from the resin (Figure 2 and 3).
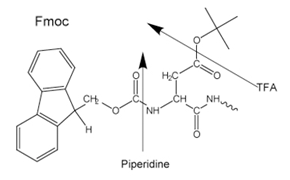
Figure 3.Side-chain protecting groups for Fmoc solid-phase peptide synthesis (SPPS)
Most peptides are prepared by the Fmoc method as the final cleavage and deprotection is carried by treatment with trifluoroacetic acid as opposed to the Boc method which requires use of highly toxic, corrosive liquid anhydrous HF in specialist equipment.
Peptides of 50 amino acids can be routinely prepared although the synthesis of proteins of over 100 amino acid are commonly reported. Longer proteins can be made by native chemical ligation of fully deprotected peptides in solution. With this method, it is possible to synthesize natural peptides that are difficult to express in bacteria, to incorporate unnatural or D-amino acids, and to generate cyclic, branched, labelled, and post-translationally modified peptides.
Liquid-phase peptide synthesis, usually utilizing Boc or Z-amino protection, has been superseded by solid-phase peptide synthesis except for existing processes of large-scale synthesis of peptides for industrial purposes.
Visit our document search for data sheets, certificates and technical documentation.
Related Articles
- Novabiochem® offers a wide range of linkers and derivatized resins for Fmoc solid-phase peptide synthesis with specialized protocols.
- Chromogenic and fluorogenic derivatives are invaluable tools for biochemistry, having numerous applications in enzymology, protein chemistry, immunology and histochemistry.
- Aspartimide formation 1,2 is caused by repeated exposure of aspartic acid-containing sequences to bases like piperidine and can result ultimately in the generation of 9 different by-products.
- Novabiochem® offers polymer supports for solid phase peptide synthesis, suitable for various peptide lengths and sequences.
- Long peptide purification removes impurities effectively, crucial for research and pharmaceutical applications.
- See All (11)
Related Protocols
- A guide to create solvent systems used for the thin-layer chromatography assay of Novabiochem products.
- Overcome challenges in synthesis and disulfide bond formation with protocols for Fmoc solid-phase peptide synthesis of peptides with cysteine and methionine.
- We provide an overview of our available reagents, together with recommendations and details of their use for synthesis of peptides containing post-translationally modified amino acids.
- Anhydrous HF is the preferred reagent for peptide cleavage from Boc-based resins, versatile and effective for various peptide synthesis.
- Fmoc resin cleavage and deprotection are crucial steps for peptide synthesis, yielding the desired peptide after resin detachment.
- See All (7)
Find More Articles and Protocols
How Can We Help
In case of any questions, please submit a customer support request
or talk to our customer service team:
Email custserv@sial.com
or call +1 (800) 244-1173
Additional Support
- Chromatogram Search
Use the Chromatogram Search to identify unknown compounds in your sample.
- Calculators & Apps
Web Toolbox - science research tools and resources for analytical chemistry, life science, chemical synthesis and materials science.
- Customer Support Request
Customer support including help with orders, products, accounts, and website technical issues.
- FAQ
Explore our Frequently Asked Questions for answers to commonly asked questions about our products and services.
To continue reading please sign in or create an account.
Don't Have An Account?