Staudinger Ligation
A High-Yield, Chemoselective, and Mild Synthetic Method
Azides react readily with triarylphosphines to form the corresponding iminophophoranes (aza-ylides) with loss of nitrogen. This transformation, originally reported by Staudinger and Meyer,1 takes place under mild conditions and leads to excellent yields of the aza-ylide products. The initially formed iminophosphorane product can undergo a number of synthetically important reactions with a variety of electrophiles, such as aldehydes and ketones. However, in aqueous medium, it is hydrolyzed to the amine product.2,3 The overall reduction of the azide to the amine is believed to take place as shown in Scheme 1.4

Scheme 1.
When an ester group is situated ortho to the phosphorus, a covalent amide bond can be generated prior to hydrolysis (Scheme 2),4 which allows this transformation to have useful applications in chemical biology.

Scheme 2.
The reaction’s high chemoselectivity and the fact that the azide and the phosphine are chemically orthogonal to most functional groups found in biological systems have given rise to a strong renewed interest in the reaction as part of a methodology to investigate cellular processes in chemical biology.3 This is perhaps best exemplified by the studies of Professor Carolyn Bertozzi of the University of California, Berkeley. Bertozzi’s research employs appropriately substituted triphenylphosphines, as reducing and tagging agents, and a sequence of reactions to produce amide-linked, unnatural, cell-surface sialosides. Metabolically generated sialosides allow the modulation of viral infection and interfere with cancer cell metastasis. Bertozzi’s approach is illustrated in Scheme 3.5
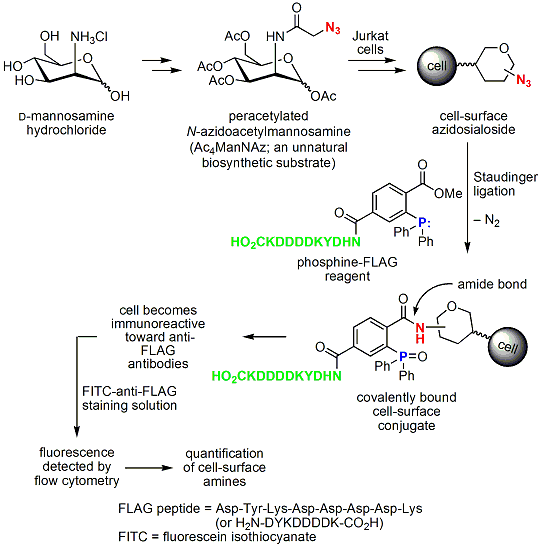
Scheme 3.
We offer many of the labeling and staining reagents commonly used in chemical biology research today, including the 1-methyl 4-pentafluorophenyl diester of 2-(diphenyl-phosphino)terephthalic acid (679011) and fluorescein 5-isothiocyanate (FITC, F2502). In addition, it also offers stable aza-ylides, such as N-(triphenylphosphoranylidene)aniline (324876).


References
如要继续阅读,请登录或创建帐户。
暂无帐户?