Guanylation of Amines by Bis(tert-butoxycarbonyl)thiopseudourea, Polymer-Bound
The synthesis of guanidine-containing bioactive molecules is of great interest in medicinal chemistry. The guanidine moiety is fully protonated under physiological conditions due to its strong basicity.1 Introduction of a guanidinyl group can be achieved by the reaction of substituted amines with various guanylation reagents.2,3,4 One such guanylating reagent is bis(tert-butoxycarbonyl)thiopseudourea, polymer-bound.
Typical Experimental Procedure
Synthesis of N,N Disubstituted- N’,N’’-bis(tert-butoxycarbonyl) Guanidine
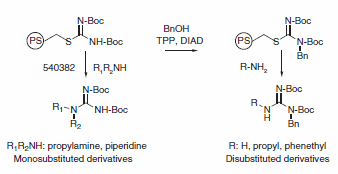
The amine (1 mmol) and bis(tert-butoxycarbonyl)thiopseudourea, polymer-bound (5 mmol) are mixed in THF (5 mL) and stirred for 40 h at room temperature. The mixture is filtered and the resin washed with THF (2 × 3 mL). The combined washings are evaporated to dryness to give a pure monosubstituted guanidine. The structures are confirmed by 1H NMR and mass spectrometry.
N,N Disubstituted-N’,N’’-bis(tert-butoxycarbonyl) Guanidine
Synthesis of N,N’ Disubstituted-N’,N’’-bis(tert-butoxycarbonyl) Guanidine
Polymer-bound bis(tert-butoxycarbonyl)thiopseudourea (200 mg, 0.2 mmol) and triphenylphosphine (313 mg, 1.2 mmol) were added to a fritted reaction vessel. A solution of benzyl alcohol (108 mg, 1.0 mmol) in THF (3 mL) was added. The mixture was stirred for 20 min, followed by dropwise addition of diisopropyl azodicarboxylate (0.196 mL, 1.0 mmol). The resulting mixture was stirred overnight at room temperature. The reaction solution was drained and the resin washed with several portions of THF and methanol.
Ammonia (2 mmol) or amine (0.6 mmol) in THF (2.5 mL) was added to the reaction vessel. The mixture was agitated overnight at room temperature. The reaction mixture was filtered and the solution collected. The resin was washed with several portions of THF and methanol. The combined reaction solution and washings were evaporated to dryness. The residue was purified by flash chromatography (silica gel, column size: 1.5 × 20 cm). The structures of the isolated products were confirmed by 1H NMR and mass spectrometry. The BOC group can be removed using standard deprotection methods to yield the desired unprotected substituted guanidine.
N,N’ Disubstituted-N’,N’’-bis(tert-butoxycarbonyl) Guanidine
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?