Solid State Synthesis
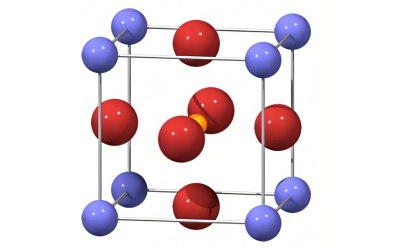
Solid-state synthesis, or the ceramic method, is commonly used to cause a chemical reaction from solid starting materials to form a new solid with a well-defined structure. End products include polycrystalline materials, single crystals, glasses, and thin-film materials that are widely used for energy and electronic applications.
Fine-grain metal compounds are combined, pelletized, and heated at a controlled temperature for a specific time period. Some metal compounds, such as metal oxides or salts, require extreme conditions, such as high temperatures and pressure, to initiate reactions in a molten flux or a rapidly condensing vapor phase. This process is often referred to as “shake and bake” or “heat and beat” chemistry.
Featured Categories
We offer a wide range of high-purity salts, including nitrates, oxalates, halides, sulfates, carbonates, and acetates, available in both anhydrous and hydrated forms.
We offer an extensive range of high-purity metals and alloys in diverse compositions and particles size, even customized for your research and production applications.
We offer an extensive range of high purity oxides and ceramics with specialized synthesis and purification techniques, and distinct particle sizes.
We offer a comprehensive portfolio of inorganic and metallic nanomaterials, functionalized nanoparticles, and nanomaterial kits for your research needs.
The reaction rate in solid state synthesis is particularly important to characterize. Solid state reactions must go to completion, as techniques for purification of formed solids are severely limited. The rate of the solid-state reaction depends on the reaction conditions, including the structural properties, shape and surface area of the reactants, the diffusion rate, and the thermodynamic properties associated with the nucleation/reaction. The chemical and physical properties of the final materials are determined by the chemical precursors and preparation techniques.
Modern preparation techniques for solid state are not limited to variations on the ceramic method. In solid state metathesis, reactions of metal compounds are initiated by an energy source (e.g., flame, ball mill) and propagated by the heat released during the formation of products and byproducts. Sol-gel methods utilize a concentrated or colloidal solution (the ‘sol’), which is sequentially heated, dried, and aged to form gels, coatings, and nanomaterials. Solvothermal methods involve heating solutions in a pressurized, closed vessel at temperatures above the standard boiling point of the chosen organic solvent; if the solvent is water, this is called the hydrothermal method. Many synthetic methods where a solid material is formed, such as vapor-phase depositions, intercalation, single crystal growth, and nanomaterial syntheses, can be classified as solid state synthesis.
Visit our document search for data sheets, certificates and technical documentation.
Related Articles
- Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the provided chart.
- Learn how activated carbon is working as a catalyst support and its advantage.
- The three types of magnetic behaviors are paramagnetism where unpaired electrons are random, ferromagnetism where unpaired electrons align, and antiferromagnetism where unpaired electrons align opposite of one another.
- Today, near-room-temperature refrigeration is almost entirely based on a vapor-compression refrigeration cycle.
- Gas sorption analysis aids in materials science and consumer product development.
- See All (32)
Find More Articles and Protocols
How Can We Help
In case of any questions, please submit a customer support request
or talk to our customer service team:
Email custserv@sial.com
or call +1 (800) 244-1173
Additional Support
- Chromatogram Search
Use the Chromatogram Search to identify unknown compounds in your sample.
- Calculators & Apps
Web Toolbox - science research tools and resources for analytical chemistry, life science, chemical synthesis and materials science.
- Customer Support Request
Customer support including help with orders, products, accounts, and website technical issues.
- FAQ
Explore our Frequently Asked Questions for answers to commonly asked questions about our products and services.
To continue reading please sign in or create an account.
Don't Have An Account?



