Pre-clinical

Pre-clinical testing establishes that the target compound demonstrates the safety and efficacy necessary to advance to human testing. At this stage, in vitro and in vivo testing can require several years and if successful, leads to the preparation and filing of an Investigational New Drug (IND) application.
Featured Categories
Browse our cell culture products for manufacturing, including our cell culture media, cell culture ingredients and supplements, bioreactors, and clarification filters.
Explore our process solution buffer and media preparation solutions, including our Mobius® single-use mixing and Mobius® FlexReady solutions.
Streamline your bioprocessing workflow: Explore our chromatography range for efficient purification. Resins, membranes, columns, and systems for every scale.
Toxicology testing services are critically important. A broad spectrum of in vitro and in vivo toxicology testing is required and must be designed in accordance with international guidelines, conducted in full compliance with good laboratory practice (GLP) regulations.
Process Development Considerations
- Cell line development
- Clone selection
- Media and feed screening
- Upstream process development
- Master cell bank establishment
- Downstream process development
- Formulation development
- Analytical methods development and optimization
- Analytical process development support
- Biosimilars comparability analytical programs
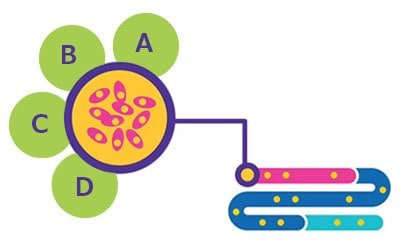
Figure 1.Cell Line Development. A. BioReliance® Product Characterization. B. BioReliance® Cell Banking & Storage. C. BioReliance® Cell Line Development. D. BioReliance® Cell Line Characterization.
Cell Line Development Considerations
- Clone choice – cell lines that can produce the biologic at a sufficient titer and quality
- Robustness studies – studies that replicate scale-up conditions
- Genetic stability – protein production and quality should remain stable after >60 generations of cells
Process Development Considerations
- Process efficiency – eliminating wasteful steps and capacity utilization
- Process viability – reproducibility through scale-up and tech transfer
- Financial viability – balancing productivity with cost
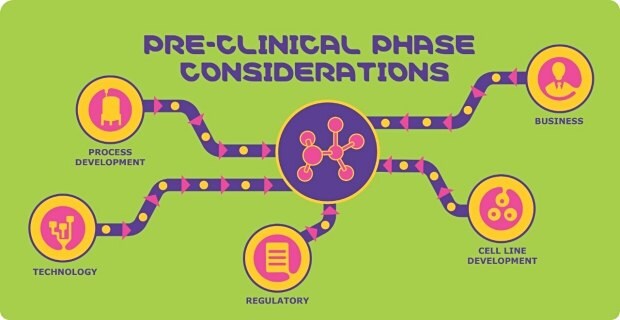
Technology Considerations
- Flexibility – single-use equipment enhances flexibility, templating, and rapid suite configuration, and reduces cleaning costs
- Scalability – when choosing technologies at the earliest stages, such as scaling a bioreactor used for mammalian cell culture from 3 L to 200 L to 2000 L
- Ease of use – solutions include pre-assembled sterile process flow paths; connectors that allow for aseptic connection, disconnection, and re-connection
Regulatory Considerations
- Patient safety – clinical development milestones must assess safety and product effectiveness
- Product quality and process robustness – data collection and analysis are needed to confirm
- Filing strategy – knowing the countries where filing will happen should guide who inspects the manufacturing facility
Business Considerations
- Speed to clinic – heavy investments are being made in research and development without returns
- Process efficiency – proactively consider scale-up, not just initial speed
- Filing strategy – identify the country to file in first and rapidly obtain access to the market
Access to the right resources helps you focus on discoveries with the greatest potential to help patients in need. Select your stage of the development process to learn more or follow the product and service links for resources.
Visit our document search for data sheets, certificates and technical documentation.
Related Articles
- Raw material selection's critical role in viral vector production for gene therapy includes regulatory guidelines and supplier collaboration importance.
Biotech Resources
- FDA Investigational New Drug (IND) Application
Explore FDA’s guidance on IND applications, a crucial step in drug development for biotech startups. Navigate regulatory intricacies efficiently.
- Emerging BioTalk Blog
Your gateway to the latest news in biotech. Dive into discussions on market trends, bioprocessing, and technologies for novel modalities. Join the discussion today.
- Brochure: Integrated Bioprocess Solutions
Navigate the path to commercialization with our comprehensive guide for Biopharmaceutical Startups. Accelerate your journey with our range of tools and services.
- Biopharmaceutical Application Guide
Navigate the biopharmaceutical landscape with our application guide, providing resources and solutions for mAb, ADC, and mRNA processes.
How Can We Help
In case of any questions, please submit a customer support request
or talk to our customer service team:
Email custserv@sial.com
or call +1 (800) 244-1173
Additional Support
- Calculators & Apps
Web Toolbox - science research tools and resources for analytical chemistry, life science, chemical synthesis and materials science.
- Customer Support Request
Customer support including help with orders, products, accounts, and website technical issues.
- FAQ
Explore our Frequently Asked Questions for answers to commonly asked questions about our products and services.
Biotech Hub Resources Workflow

Discovery
Identifying the considerations, resources, and support you need to develop a new biologics candidate

Phase I–II
Accelerating your process development with tips, templates, and application guides

Phase III and Manufacturing
Progressing from scale-up and tech transfer to quality production for trials and commercialization

Startup Programs
Connecting with resources and grant programs that can unlock your molecule’s potential

Regulatory
Navigating one of the world’s most regulated industries starts with a trusted guide
To continue reading please sign in or create an account.
Don't Have An Account?