Choosing Essential or Classic or Advanced Level of Service
Service Descriptions
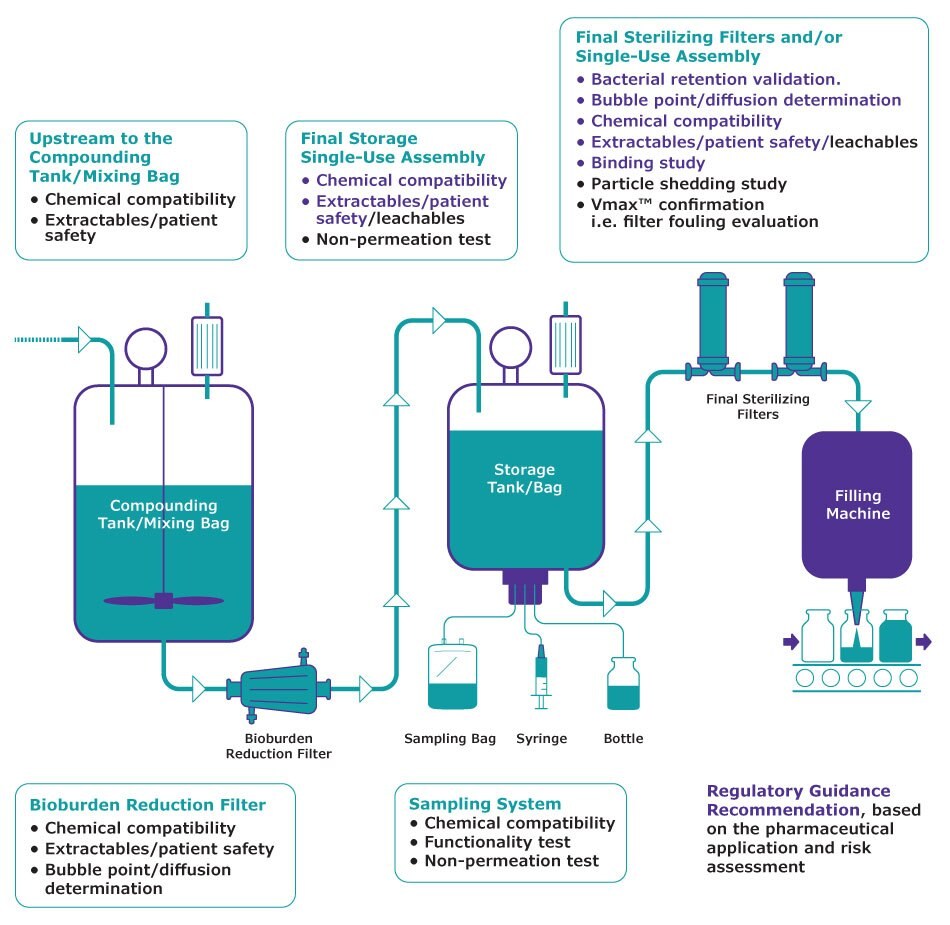
Bacterial retention validation with standard B. diminuta (BAC-Bd)
Validate the performance of your sterilizing grade filter by simulating your product and process conditions. B. diminuta is used as the standard challenge microorganism. This includes one viability test and one recovery test. These preliminary tests will assess the viability and the recovery of our bacteria in the product in order to select the most appropriate methodology for the bacterial retention test. Use of 3 membranes from 3 lots including at least one low bubble point membrane.
Bacterial retention validation with natural bioburden (BAC-Biob)
In the case you have a natural bioburden strain smaller than B. diminuta in your product, the bacterial retention can be performed with your process bioburden strain.
Extractables (EXT)
Identify and quantify the extractables which may be extracted out from the filter/single use assembly by employing the Model Solvent Stream Approach and worst-case test conditions. Analytical methods used are NVR, TOC, FTIR, RP-HPLC, LC-MS, GC-MS, ICP-MS(when applicable). A review of existing data is performed in order to assess if an extractable report can be provided. If not, actual testing is performed, and a test protocol and test report is then provided.
Patient Safety assessment (RISKL)
Assess the potential impact of the substances that have been detected, identified and quantified on patient safety. This assessment is done by a toxicologist.
Leachables (LEA)
Identify and quantify the leachables which may be leached out from the filter/single use assembly with the use of your actual product under normal processing conditions. Analytical methods used are GC-MS and/or LC-MS (when applicable).
Vmax™ Confirmation/filter fouling evaluation
Confirms a selected filter membrane area is appropriate to process a specific batch volume, in a specified time and minimum flow rate as determined by a prior Vmax™ study. This study determines the filter safety factor and provides conclusion on filter fouling.
Integrity test: bubble point determination (BP)
Bubble point testing is designed to provide a product-specific minimum bubble point value. The bubble point ratio test is using 3 membranes from the same lot and 1 product lot or 9 membranes from 3 lots and 3 product lots.
Integrity test: diffusion determination (DIF)
Diffusion testing is designed to provide a product-specific maximum diffusion value. The diffusion ratio test is using 3 filter devices from the same lot and 1 product lot or 9 filter devices from 3 lots and 3 product lots.
Integrity test with a rinsing fluid (RIN)
Determine the post-use minimum bubble point or/and diffusion value of a filter device, wetted with product and then rinsed out with rinsing fluid.
Compatibility study (COM)
Assess the chemical compatibility of a filter device/single use assembly based on key characteristics, after prolonged exposure with the drug product. Provide evidence that the process fluids and conditions do not adversely impact the structure of the filter device/single use assembly. A specific test is performed or if possible, a compatibility report based on bibliographic data can be provided.
Particle shedding (PSH)
Provides particle shedding data (particle size of ≥ 10 µm and ≥ 25 µm) on 3 devices with the drug product and using actual and scale-down customer worse-case filtration conditions.
Binding study (BIND)
Show the filter does not remove unacceptable amounts of stream compounds. One lot of product and 3 membranes from 3 lots are tested by simulation of your process conditions. Several samples are taken and sent back to you for active or preservative or any other ingredient analysis.
Compatibility certificate
Demonstrate the chemical compatibility of a Filter device/single use assembly, towards your Drug product according to worst case process parameters, based on bibliographic research (qualification documents & compatibility charts & literature). A certificate report is provided. No test protocol is provided.
Non-Permeation test for Bags
Demonstrates that the bags are not permeable to disinfectant/decontamination agents. Fertility test on 6 ATCC strains after 3 lab-scale simulations.
Functionality test
Verify that the Sampling element is within the qualified acceptance criteria after product contact and process conditions simulation.
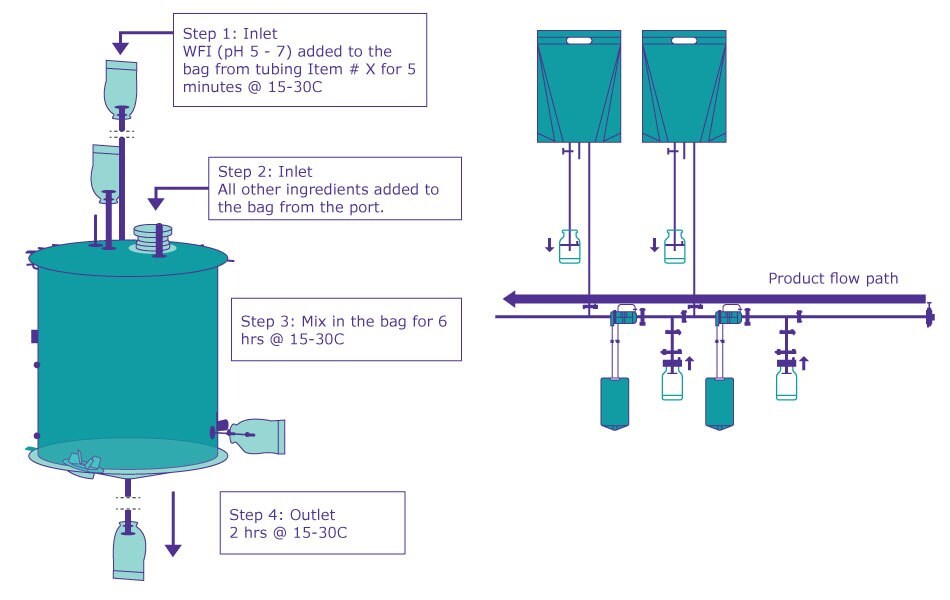
Example Mobius® Assembly Drawings with Product Flow Path
Choosing Essential or Classic or Advanced Level of Service
Essential: Rely on us for guidance. We will follow a streamlined process to complete your validation study without changing quality and regulatory requirements.
Classic: Rely on us for guidance. We'll do what's required to get your validation study done — with the highest quality standards.
Advanced: Let’s collaborate. We'll discuss your specific requirements and customize a validation plan that works for your advanced needs and complex studies.
Below are the defined study parameters for each level of service. For more detailed descriptions by test type, download the comprehensive data sheet.
Service Level Comparison Chart
To continue reading please sign in or create an account.
Don't Have An Account?