Recommended Standard Method for Isolating Mononuclear Cells
Cell separation using Ficoll-Paque products can be carried out over a wide range of blood sample volumes. With its high yield, this method can be adapted to the processing of very small amounts of blood, such as may be obtained from children. For maximum reproducibility of separation it is recommended that a standardized procedure be used. The following procedure has been evaluated in our laboratories with Ficoll-Paque PLUS and is recommended for separation of normal blood samples. Simple changes can easily be made to suit a particular centrifugation system. The same procedure is recommended when separating cells using Ficoll-Paque PREMIUM, Ficoll-Paque PREMIUM 1.084, and Ficoll-Paque PREMIUM 1.073.
To standardize the technique, blood volume and diameter of the centrifuge tube should be chosen first. These factors determine the height of the blood sample in the tube and consequently the centrifugation time. Increasing the height of the blood sample in the tube increases red cell contamination. The separation is, however, not appreciably affected by changing the diameter of the tube. Hence a larger volume can be separated with the same degree of purification in a tube of larger diameter if the height of the blood sample in the tube and the separation time are kept constant.
The yield and degree of purity of the mononuclear cells depend to a considerable extent on the efficiency of red cell removal.
When erythrocytes in whole blood are aggregated, some mononuclear cells are trapped in the clumps and therefore sediment with the erythrocytes. This tendency to trap mononuclear cells is reduced by diluting the blood. Dilution gives a better yield of mononuclear cells and reduces the size of the red cell clumps. Aggregation of erythrocytes is enhanced at higher temperatures (37 °C), which consequently decreases the yield of mononuclear cells. At lower temperatures (4 °C); however, the rate of aggregation is decreased but the time of separation is increased, which also decreases the yield of mononuclear cells. A compromise temperature of 18 ºC to 20 °C gives optimal results.
Equipment and solutions required but not provided
- Sterile balanced salt solution or other standard salt solutions (see Preparation of reagents).
- Centrifuge with swing-out rotor (brake should be off).
- Sterile centrifuge tubes and pipettes.
- Sterile needles and syringes.
- Red blood cell lysis solution of choice (if isolating granulocytes).
Preparation of reagents
Diluent and washing solution
The balanced salt solution for dilution of the blood and cell washing can be made according to the instruction below. Other diluents and washing fluids such as isotonic Ca2+/Mg2+ free phosphate buffered saline (e.g. Dulbecco’s PBS), salt solutions (e.g., Hank’s) or cell culture media (e.g., RPMI 1640) may also be used.
Balanced salt solution
To prepare the balanced salt solution, mix one volume stock solution A with nine volumes stock solution B and sterilize. At least 20 mL for each sample should be processed. Other sterile standard salt solutions may also be used.
Dissolve in approximately 950 mL of distilled water and add concentrated HCl until the pH is 7.6 before adjusting the volume to 1L.
To prepare the balanced salt solution, mix 1 volume of solution A with 9 volumes of solution B. Prepare the solution freshly each week. Other standard salt solutions may be used.
Ficoll-Paque product
Warm the Ficoll-Paque density gradient media to 18 °C to 20 °C before use. For samples larger than 3 mL, see Notes.
Preparation of the sample
Fresh blood should be used to ensure high viability of isolated mononuclear cells. Prepare the sample at 18 ºC to 20 °C.
- To a 10 mL centrifuge tube, add 2 mL of defibrinated- or anticoagulant-treated blood and an equal volume of balanced salt solution (final volume 4 mL).
- Mix the blood and buffer by inverting the tube several times or by drawing the mixture in and out of a pipette.
Procedure for isolation of mononuclear cells
- Invert the Ficoll-Paque media bottle several times to ensure thorough mixing.
For withdrawal of Ficoll-Paque media by syringe: Snap-off the polypropylene cap and insert the syringe needle through the septum (Figure 1).
For withdrawal of Ficoll-Paque media by pipette: Remove the snap-off polypropylene cap. Lift the aluminum ring. Pull off the metal seal. Remove the silver ring.
Remove the rubber closure. Using aseptic techniques, withdraw the required volume of Ficoll-Paque media (Figure 2). - Add Ficoll-Paque media (3 mL) to the centrifuge tube.
- Carefully layer the diluted blood sample (4 mL) onto the Ficoll-Paque media solution (Figure 3).
Important: When layering the sample do not mix the Ficoll-Paque media solution and the diluted blood sample. - Centrifuge at 400 g for 30 to 40 min at 18 ºC to 20 °C (brake should be turned off).
- Draw off the upper layer containing plasma and platelets using a sterile pipette, leaving the mononuclear cell layer undisturbed at the interface (Figure 4 and Figure 5). The upper layer, which contains the plasma, may be saved for later use.
- Transfer the layer of mononuclear cells to a sterile centrifuge tube using a sterile pipette.

Figure 1.Polypropylene cap and silver ring removal
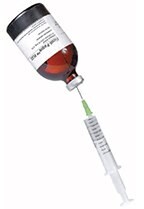
Figure 2. Withdrawal of Ficoll-Paque media
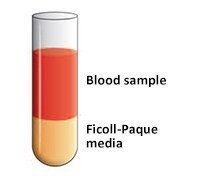
Figure 3.Blood sample layered onto Ficoll-Paque media
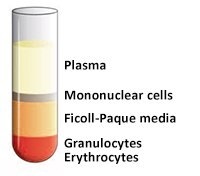
Figure 4.Prior to removal of the upper layer.
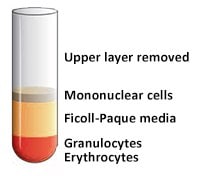
Figure 5.After the upper layer (Plasma) removed.
Washing the cell isolate
- Estimate the volume of the transferred mononuclear cells. Add at least 3 volumes (~ 6 mL) of balanced salt solution to the mononuclear cells in the centrifuge tube.
- Suspend the cells by gently drawing them in and out of a pipette.
- Centrifuge at 400 to 500 × g for 10 to 15 min at 18 °C to 20 °C.
Note: A centrifugation at high speed increases the mononuclear cell recovery. However, if it is important to also get rid of platelets a lower centrifugation speed is recommended (60 to 100 × g).
- Remove the supernatant.
- Resuspend the mononuclear cells in 6 to 8 ml balanced salt solution.
- Centrifuge at 400 to 500 × g (or 60 to 100 × g for removal of platelets) for 10 min at 18 °C to 20 °C.
- Remove the supernatant.
- Resuspend the cell pellet in media appropriate for the application.
Procedure for isolation of granulocytes
- Perform density gradient centrifugation using Ficoll-Paque media as described above in Procedure for isolation of mononuclear cells, steps 1 to 6.
- Draw off the upper layer of Ficoll-Paque media using a sterile pipette, leaving the white cell layer of granulocytes above the red blood cell layer undisturbed.
- Collect the thin white cell layer of granulocytes with a pipette and transfer to a sterile centrifuge tube.
- Resuspend the cells in at least five volumes of balanced salt solution and centrifuge at 400 × g for 15 min.
- Lyse remaining red blood cells with any red blood cell lysis solution of choice.
- Centrifuge the granulocytes at 400 to 500 × g for 10 to 15 min at 18 °C to 20 °C.
- Remove the supernatant.
- Resuspend the granulocytes in 6 to 8 ml balanced salt solution.
- Centrifuge at 400 to 500 × g for 10 min at 18 °C to 20 °C.
- Remove the supernatant.
- Resuspend the cell pellet in media appropriate for the application.
Notes
Anticoagulants: Heparin, EDTA, citrate, acid citrate dextrose (ACD), and citrate phosphate dextrose (CPD) may be used as anticoagulants for the blood sample. Defibrinated blood requires no anticoagulant. Defibrination, however, results in a lower mononuclear cell yield and may cause increased contamination by red cells3. It also causes selective loss of monocytes.
Bøyum has found that a slightly purer mononuclear cell preparation is obtained using EDTA instead of heparin as anticoagulant3. It has also been noted in the purification of mononuclear cells from sources other than peripheral blood that addition of heparin may cause gelling of cell suspensions4. Citrate-stabilized blood may result in better quality RNA and DNA than other anticoagulants, and produce a higher yield of mononuclear cells. Heparin affects T-cell proliferation and binds to many proteins. EDTA is good for DNA based assays, but influences Mg2+ concentration and causes problems for cytogenetic analysis5. It has also been shown that the RNA yield is higher in EDTA-blood than in heparin-blood6.
Blood volume: Larger volumes of blood may be processed with the same efficiency of separation by using centrifuge tubes of increased diameter while maintaining approximately the same heights of Ficoll-Paque media (2.4 cm) and blood sample (3.0 cm) as in the standard method described above. Increasing the tube diameter does not affect the separation time required.
Blood sample storage: Blood samples should be free of clots and processed as soon as possible after collection to ensure optimal results. Delays in processing the blood can result in loss of viability, lower cell recoveries and more contaminating granulocytes and/or erythrocytes. Indeed, storage for 24 h at room temperature has been reported to result in reduced lymphocyte yield, altered expression of surface markers, and reduced response to mitogenic stimulation 5,7,8. Dead cell removal: Dead cells are removed during cell isolation using Ficoll-Paque PLUS9,10,58.
Density and temperature: The temperature at which density gradient separations are carried out are naturally affected by room temperature, centrifuge temperature, temperature of the density gradient media, and temperature of the liquid sample. There can, at times, also be confusion regarding what is meant by room temperature (e.g., in Europe it can be 20 ºC and in North America > 20 ºC). It is known that the densities of Ficoll-Paque products decrease with increase in temperature. For instance Ficoll-Paque PREMIUM (1.077 g/ml) exhibits a density of 1.0772, 1.0767, and 1.0758 at 20 ºC, 22 ºC, and 25 ºC, respectively.
Pathological blood samples: The standard method described above has been developed for the purification of mononuclear cells from peripheral blood of normal, healthy, human donors. Different results may be obtained with samples taken from donors with infections or other pathological conditions, such as cancer (see Further applications of Ficoll-Paque PLUS and Ficoll-Paque PREMIUM, page 12).
Platelet removal: If it is important to remove all platelets from the mononuclear cell fraction a second centrifugation in a 4% to 20% sucrose gradient layered over Ficoll-Paque PLUS can be applied. This procedure will effectively remove any platelet contamination11. Platelets will remain at the top of the sucrose gradient and mononuclear cells will sediment through the sucrose gradient to the top of the Ficoll-Paque PLUS layer. Alternatively, the platelets may be removed by aggregation with adenosine-5’-diphoshate (ADP) before separating the mononuclear cells12.
Troubleshooting inadequate performance
If used according to the recommended standard procedure, Ficoll-Paque PLUS and Ficoll-Paque PREMIUM may be expected to give trouble-free isolation of human peripheral blood mononuclear cells with results as shown on page 2. As mentioned earlier, Ficoll-Paque PREMIUM 1.073 and Ficoll-Paque PREMIUM 1.084 will lead to isolation of cell preparations having slightly different density subsets of mononuclear cells. However, deviations in certain experimental parameters may lead to poor results and the troubleshooting chart given here is intended to assist in the rapid identification and correction of the problem causing reduced performance.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?