radiello® Diffusive Air Sampling Application - Nitrogen and Sulfur Dioxide
radiello Components Used:
Blue diffusive body Product No. RAD1201
Supporting plate Product No. RAD121
Vertical adapter Product No. RAD122 (optional)
Chemiadsorbing cartridge Product No. RAD166
Principle
The cartridge, Product No. RAD166, is made of microporous polyethylene coated with triethanolamine (TEA). Nitrogen (NO2) and sulfur (SO2) dioxide is chemiadsorbed onto TEA as nitrite and sulfite or sulfate ions respectively. Nitrite is quantified by visible spectrophotometry while sulfite and sulfate are analysed by ion chromatography (NO2 and SO2 can be analysed together by ion chromatography).
Sampling is selective for gaseous molecules: any airborne nitrite, sulfite or sulfate will not cross the diffusive membrane.
Sampling Rates
NO2
The sampling rate value Q at 298 K (25°C) and 1013 hPa is 0.141 ± 0.007 ng·ppb-1·min-1.
SO2
The sampling rate value Q at 298 K (25°C) and 1013 hPa is 0.466 ± 0.022 ng·ppb-1·min-1.
Effect of Temperature, Humidity, and Wind Speed
Sampling rate of NO2 varies from the value at 298 K on the effect of temperature (in Kelvin) following the equation:
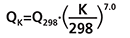
where QK is the sampling rate at the temperature K ranging from 263 to 313 K (from -10 to 40 °C) and Q298 is the reference value at 298 K.
Sampling rate for SO2 does not vary with temperature between 263 and 313 K (from -10 to 40 °C).
Sampling rate is invariant with humidity in the range 15 - 90% and with wind speed between 0.1 and 10 m·s-1 for both gases.
Calculations
NO2
The concentration CNO2 is calculated according to the equation:
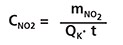
where mNO2 is nitrite mass in ng found on the cartridge, t is exposure time in minutes and QK is the sampling rate value at the temperature K in Kelvin.
SO2
Convert the sulfite found onto the cartridge into sulfate by multiplying its mass by 1.2, then sum the obtained value to the sulfate found in the cartridge. The concentration in ppb is calculated according to the equation:
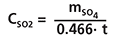
where mSO4 is the overall sulfate mass in ng found in the cartridge (sulfate itself and sulfite converted into sulfate) and t is exposure time in minutes.
USER TIP
It is advisable to measure the sampling temperature by the thermometer Product No. RAD126.
Exposure
Exposure up to 15 days is feasible but if relative humidity is higher than 70% for the entire sampling duration it is not advisable to sample for more than 7 days. Due to the fact that TEA is very hygroscopic in fact, even if water does not actually interfere with sampling or analysis, the excess water adsorbed by the cartridge could cause some loss of adsorbing medium by percolation.
Limit of Quantitation and Uncertainty
Sampling rate of NO2 and SO2 is linear ranging from 10,000 to 5,000,000 ppb·min. Limit of quantitation after 7 days exposure is 1 ppb for both gases. The uncertainty at 2σ is 11.9% for NO2 and 9.2% for SO2.
Storage
The cartridges are stable for at least 12 months before and 4 months after the sampling, if kept in the dark at 4 °C.
Expiration date is printed on the plastic bag.
Do not expose all of the cartridges belonging to the same lot, keep at least two of them as blanks.
Analysis
Add 5 mL of water in the plastic tube with the cartridge and stir vigorously by a vortexer for 1 minute. Do the same with two-three unexposed cartridges.
Colorimetric Determination of Nitrite ion
Nitrogen dioxide is quantitatively converted to nitrite ion. Prepare the following reactives:
- Sulfanilamide: dissolve 10 g of sulfanilamide in 100 mL concentrated HCl and dilute to 1,000 mL with water
- Dissolve 250 mg of N-(1-naphthyl)ethylendiamine dihydrochloride in 250 mL of water (discard the solution when it turns brown).
Transfer 0.5 mL (or a different volume, see the table below) of the cartridge extraction solution to a plastic or glass 10 mL tube along with 5 mL of sulfanilamide reactive. Cap tigthly, stir and wait for 5 minutes. Add 1 mL of NEDA reactive, stir and wait for 10 minutes. Do the same with unexposed cartridges.
Measure the absorbance of samples at 537 nm using water to zero the spectrophotometer, then subtract the blank value from unexposed cartridges. Prepare the calibration standards in the same way from sodium nitrite solutions of concentration ranging from 0.1 to 20 mg·l-1 expressed as NO2-.
When nitrite ion concentration is higher than 20 μg·mL-1 (corresponding to 7 days of exposure to 70 ppb) the absorbance value is no longer comprised in the calibration curve. To analyse the samples, draw smaller amounts of the extraction solution as shown in the table. In order to mantain the overall volume unaltered, add the listed volume of water.
Determination of the Sulfite and Sulfate ions
Though SO2 is converted into sulfite and sulfate ions with variable ratios, the sum of the two ion equivalents is linear with exposure to SO2. To obtain calibration curves, prepare solutions containing both ions at concentrations ranging from 5 to 50 mg·l-1. Perform the ion chromatography analysis of the standard solutions and the extraction solutions from radiello cartridges in the same way according to your usual laboratory practice.
Materials
To continue reading please sign in or create an account.
Don't Have An Account?