Pull-down Assays
Pull-down assays involve isolation of a protein complex by adsorbing the complex onto beads. Immobilized ligands on the beads bind specifically to a component of the complex, either via an affinity tag (e.g., GST, histidine, maltose binding protein, etc.) or an antibody. Pull-down assays are often used for the isolation of low µg amounts of complexes, mainly for the purpose of identifying individual subunits. They have also been used to isolate material for limited functional studies, but other methods appear more suitable for the production of larger (mg) amounts. The components of the captured complex are often eventually analyzed by mass spectrometry to identify the subunits.
Pull-down assays are important tools for mapping protein-protein interaction networks. They have been successfully used on a global scale to map protein-protein interactions in a number of organisms (e.g., yeast, E. coli, C. elegans). Genetic approaches involving the yeast-two-hybrid (Y2H) system and similar technologies are useful complements to multiprotein complex isolation. False positives are a major concern associated with mapping protein-protein interactions on a global scale. Therefore, in addition to the relevant control experiments, it is useful to perform both complex isolation and Y2H experiments independently to validate data sets. This can be followed by verification in a more in vivo-like milieu.
This section covers three different pull-down methods for isolating small amounts of complexes for the purpose of identifying components of the complex. Two of the methods involve the affinity tagging of one (known or supposed) component of the complex. The third method, co-immunoprecipitation, involves the use of an antibody directed towards one (known or suspected) component. All three methods use beads with appropriate ligands to pull down the complex from a crude biological sample. An overview of the pros and cons of the different methods is shown in Table 2.1.
Affinity pull-down assay
Affinity pull-down assays are well established for the isolation and subsequent identification of protein complexes. GST pull-down assays involve affinity purifications of one or several unknown proteins from a biological sample using a GST-tagged bait protein. The basic principle is that the GST-tagged bait protein binds to its partners, and the resulting complex is captured on beads with immobilized glutathione. A control is included to identify false positives that bind to GST in the absence of a bait protein. The control can either be lysate from separately transformed cells that express GST (not the bait fusion protein) or lysate from non-transformed cells to which GST is added. Design of the appropriate controls for the experiment is essential.
A common assay format is to spin down the beads and the bound proteins with a centrifuge, hence the term “pull-down”. The principle is shown in Figure 2.4.
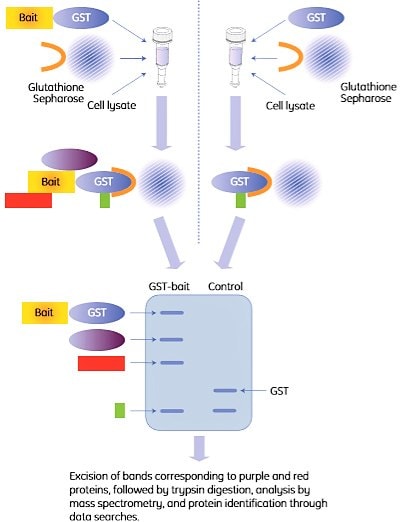
Figure 2.4. Outline of a GST pull-down experiment. The right-hand procedure shows the control experiment. In this example, two proteins (purple and red) were identified by SDS-PAGE as interaction partners with the bait protein (left lane). One additional protein (green) was pulled down but identified as a false positive (GST binder) by the control (right lane).
GST pull-down assay using GST SpinTrap Purification Module
Materials
GST SpinTrap Purification Module containing 10× PBS, MicroSpin columns, dilution buffer, and reduced glutathione
Buffer preparation
1× PBS: To prepare 1× PBS, dilute the supplied 10× PBS ten-fold with sterile water. Store at 4 °C. Final concentration will be 10 mM phosphate, 2.7 mM KCl, 140 mM NaCl, pH 7.3.
Dilution buffer: as supplied, 50 mM Tris-HCl, pH 8.0
Elution buffer: 10 mM reduced glutathione, 50 mM Tris-HCl, pH 8.0. To prepare elution buffer, pour the entire 50 mL volume of dilution buffer supplied with the module into the bottle containing the reduced glutathione (0.154 g). Shake until completely dissolved. Store as 1 to 20 mL aliquots at -20 °C.
Procedure
The amount of lysate required to enable the detection of protein complex components varies. A useful starting point is a lysate volume corresponding to 106-107 tissue culture cells. Use a second column to perform a control experiment using a cell lystate with GST only (no bait fusion protein).
- Resuspend Glutathione Sepharose 4B in each SpinTrap column by vortexing gently.
- Loosen the column caps one-fourth turn. Remove (and save) bottom closures.
- Place each column into a clean 1.5 or 2 mL microcentrifuge tube. Centrifuge at 735 × g for 1 min.
- Discard the buffer from each centrifuge tube and replace the bottom closures.
- Apply up to 600 µL of lysate containing the GST-fusion (bait) protein to a column. For a control, apply the same volume of lysate with GST (no bait protein).
- Recap each column securely and mix by gentle, repeated inversion. Incubate at 4 °C for 2 h.
- Remove (and save) the top caps and bottom closures. Place each column into a clean, pre- labelled 1.5 or 2 mL microcentrifuge tube.
- Centrifuge at 735 × g for 1 min to collect the flowthrough. Save the flowthrough for analysis by SDS-PAGE.
- Place each column into a clean, prelabeled 1.5 or 2 mL microcentrifuge tube.
- Apply 600 µL of 1× PBS wash buffer to each column and repeat the centrifugation procedure. Additional 600 µL washes with 1× PBS can be performed if desired. It is important to optimize this step for good results.
- Add 100 to 200 µL of elution buffer to each column. Replace top caps and bottom closures. Incubate at room temperature for 5 to 10 min. It is important to optimize this step for good results.
- Remove and discard top caps and bottom closures and place each column into a clean 1.5 or 2 mL microcentrifuge tube.
- Centrifuge all columns and collect the eluates. Analyze by SDS-PAGE.
The selection of buffer conditions could affect protein-protein interactions.
It is necessary to optimize wash conditions for each experiment.
Poor recovery of a complex may be due to that the affinity tag is not accessible for binding to the affinity ligand when the complex has been formed. In such cases, it is recommended to change the tagging site (e.g., from N- to C-terminal) or to tag another complex component.
Depending on the scale and the required throughput, a number of options are available for GST pull-down assays (see “Materials” at bottom of this section).
Tandem affinity purification, TAP
The TAP method, with or without modifications, has been used to purify and identify complexes from yeast, trypanosomes, fruit flies, humans, and plants.
TAP differs from the approach described earlier by employing two successive affinity chromatography steps to isolate protein complexes. The rationale behind the use of two affinity steps is to enhance the specificity of the purification procedure and hence reduce the number of false positives. Furthermore, the conditions used throughout this method are mild in order to preserve complex integrity and to maximize yield. The method has been used successfully in many cases.
The basic principle behind TAP is shown in Figure 2.5. Briefly, a bait protein (a known or suspected complex component) is tagged with both protein A and calmodulin binding peptide (CBP) with a tobacco etch virus (TEV) protease cleavage site between the two tags. The double-tagged bait protein is expressed at near natural levels, since overexpression may trigger non-physiological interactions. The complex is first adsorbed to IgG Sepharose beads, via the protein A tag, and eluted by cleavage with TEV. The complex is then adsorbed to calmodulin Sepharose beads (via the CBP tag) and eluted with EGTA. The complex components are separated by SDS-PAGE and analyzed by mass spectrometry.
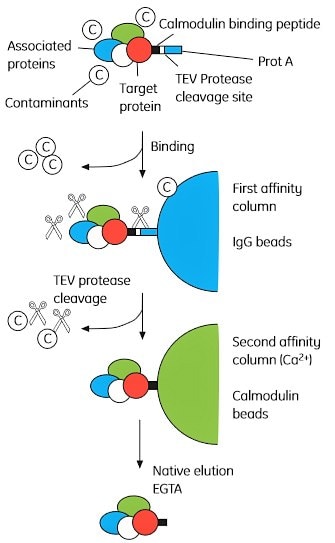
Figure 2.5. Overview of the tandem affinity purification (TAP) method. The TAP tag comprises CBP (calmodulin binding peptide) linked to a Protein A domain separated by a TEV cleavage site. Permission to reprint this figure was granted by Elsevier Global Rights Department.
In higher organisms it may be necessary to suppress the corresponding endogenous gene for the target protein by the use of RNAi technology (iTAP).
Co-immunoprecipitation
Immunoprecipitation is a well-established technique that uses antibodies to isolate antigens from crude biological samples. The name is historical and originates from analysis methods based on the precipitation reaction obtained when mixing antibody and antigen at the correct ratio. The method described here is a pull-down assay rather than a precipitation reaction. Co-immunoprecipitation uses antibodies directed towards one (known or supposed) component of a complex. The antibody binds to its antigen, which is part of a multiprotein complex. The antibody-protein complex assembly is then captured through the addition of protein A or protein G Sepharose beads to the mixture. The principle is shown in Figure 2.6.
Alternatively, an appropriate antibody can be immobilized on an activated matrix, such as NHS-activated Sepharose. Similarly, an antibody can be cross-linked to a Protein A or Protein G medium and used to co-immunoprecipitate components of protein complexes.
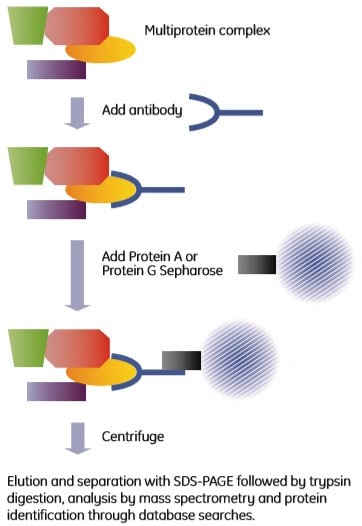
Figure 2.6. Outline of a co-immunoprecipitation experiment.
Co-immunoprecipitation with protein A or protein G
Materials Immunoprecipitation Starter Pack (contains nProtein A Sepharose 4 Fast Flow and Protein G Sepharose 4 Fast Flow)
Lysis buffers
Other buffers
PBS: 1 mM KH2PO4, 10 mM Na2HPO4, 137 mM NaCl, 2.7 mM KCl, pH 7.4
Wash buffer: 50 mM Tris, pH 8
Sample buffer: 1% SDS, 100 mM DTT, 50 mM Tris, pH 7.5 (reducing)
Media preparation
nProtein A Sepharose 4 Fast Flow and Protein G Sepharose 4 Fast Flow are supplied preswollen in 20% ethanol. Each medium must be thoroughly washed prior to use to remove the 20% ethanol storage solution. Residual ethanol may interfere with subsequent procedures.
You will need 50 µL of nProtein A Sepharose 4 Fast Flow or Protein G Sepharose 4 Fast Flow 50% suspension per sample. Make sure that the beads are properly suspended before transferring.
- Gently shake the bottle of nProtein A Sepharose 4 Fast Flow or Protein G Sepharose 4 Fast Flow to resuspend the medium.
- Use a pipette with a wide-bore tip to remove sufficient slurry and transfer the slurry to an appropriate container/tube. If needed, cut the pipette tip to create a larger opening.
- Sediment the medium by centrifuging at 12 000 × g for 20 s. Carefully decant the supernatant.
- Wash the medium by adding 10 volumes of lysis buffer (see below). Invert to mix.
- Sediment the medium by centrifuging at 12 000 × g for 20 s. Decant the supernatant.
- Repeat steps 4 and 5 for a total of three washes.
For each mL of the original slurry of medium dispensed in step 2, add 0.5 mL of lysis buffer. This results in a 50% slurry. Store at 4 ºC and mix well before use.
Binding of the antigen
- Aliquot samples (500 µL) into new microcentrifuge tubes.
- Add polyclonal serum (0.5 to 5 µL), hybridoma tissue culture supernatant (5 to 100 µL), ascites fluid (0.1 to 1 µL), or purified monoclonal or polyclonal antibodies (add the volume corresponding to 1 to 5 µg). For controls, use non-immune antibodies that are as close to the specific antibody as possible (for example, polyclonal serum should be compared with normal serum from the same species).
- Gently mix for 1 h at 4 ºC.
Precipitation of the immune complexes
- Add 50 µL nProtein A Sepharose 4 Fast Flow or Protein G Sepharose 4 Fast Flow suspension (50% slurry).
- Gently mix for 1 h at 4 ºC.
- Centrifuge at 12 000 × g for 20 s and save the pellet (the beads).
- Wash the pellet three times with 1 mL lysis buffer and once with wash buffer. Centrifuge at 12 000 × g for 20 s between each wash and carefully remove the supernatants.
Dissociation and analysis
- Suspend the final pellet in 30 µL sample buffer.
- Heat to 95 ºC and incubate for 3 min.
- Centrifuge at 12 000 × g for 20 s to remove the beads. Carefully transfer the supernatant to a clean tube.
- Add 1 µL 0.1% bromphenol blue to the supernatant.
- Analyze the supernatant by SDS-PAGE, followed by protein staining, trypsin digestion, and analysis by mass spectrometry.
Materials
To continue reading please sign in or create an account.
Don't Have An Account?