Exactly Why is Low Bleed Important for Capillary Columns?
Abstract
Capillary column manufacturers often use the term ‘low bleed’ to describe their columns. How exactly does ‘low bleed’ relate to the needs of end-users? Low detection limits, positive mass spectral identifications, and instruments that are not down for maintenance are all important daily goals for a GC analyst. Using a capillary column, such as Supelco SLB-5ms, that has a low bleed characteristic is an important step in achieving these goals.
Low Bleed = Better Signal-to-Noise Ratio = Lower Detection Limits
Signal refers to the response from an analyte passing through a detector. It is the peak you see when looking at a chromatogram. System noise refers to everything else, other than the analyte, producing a response in the detector. It is the baseline on a chromatogram. The ratio of the peak height to the baseline variability is termed the signal-to-noise ratio.
High noise level is undesirable since it makes it difficult for the integration software to adequately measure all of the peak area. The better the signal-to-noise ratio, the more area counts are obtained, resulting in the ability to achieve lower detection limits. Figure 1 shows a graphic illustration of a peak when column bleed (a significant source of system noise) is low vs. high.

Figure 1.Illustration of Signal-to-Noise Ratio
Analysts rely on GC-MS and other GC methods to provide highly sensitive, low-level detection. When measurement is required at the ppb or even ppt level, extreme care must be taken to ensure that nothing interferes with the analysis. For this reason, today’s chemists require capillary columns, such as Supelco SLB-5ms, that exhibit a very low level of bleed in addition to being inert towards the various analytes in the method.
Low Bleed = Cleaner Mass Spectra = Easier Mass Low Bleed = Cleaner Mass Spectra = Easier Mass Spectral Identifications
Analysts using MS detection have an additional concern: high column bleed that can interfere with proper mass spectral identification of analytes. In addition to the primary, or quant ion < in a mass spectrum, MS programs are set to measure the abundance of other ions that are characteristic to the analyte of interest. For the software to assign a high probability of positive identification, these so-called secondary, or qualifier ions must be within specific ratio ranges relative to the primary ion when the mass spectrum from a peak in a realworld sample extract is compared to a mass spectral library entry. If extraneous ions, such as those column bleed, are present in the mass spectrum, the software will assign a lower probability of positive identification.
Additionally, many US EPA methodologies require GC-MS analysts to assign tentative identification to non-target “unknown” compounds which may also be present in the sample extract. These are called Tentatively Identified Compounds, or TICs. In order for the software to assign a high probability of positive identification, the mass spectrum from the sample extract must compare well with the mass spectral library entry. High column bleed levels can interfere with this comparison, resulting in the reporting of TICs that are either poorly identified or misidentified.
Figures 2 and 3 show mass spectra of benzo(g,h,i)perylene at a 5 ng on-column level and an oven temperature of 325 °C. The major column bleed ion, m/z = 207, resulting from the formation of hexamethylcyclotrisiloxane (D3), should be present at a level lower than m/z = 138 and m/z = 277, two secondary ions used to confirm the identity of the peak. Figure 2 is from a Supelco SLB-5ms column. The major bleed ion, m/z = 207, is lower than both of the secondary ions. Figure 3 is from a Competitor “A” column. The major bleed ion is actually larger in size than the two secondary ions.
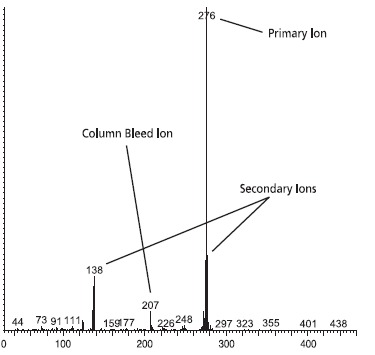
Figure 2.Mass Spectrum from SLB-5ms Column

Figure 3.Mass Spectrum from Competitor “A” Column
What does all this mean? The MS software would assign a low probability of positive identification for benzo(g,h,i)perylene for analyses using the Competitor “A” column. For TICs, the situation would be less desirable since retention time data would not be available to assist with identification.
Low Bleed = Reduced Detector Contamination = Less Instrument Downtime
High column bleed can foul the GC detector, reducing detector sensitivity. When the fouling becomes severe enough to warrant action, the detector must be dismantled and cleaned, a procedure that may remove an instrument from service for one or more days. MSD lenses can become coated and require polishing. The active foil in an ECD can become fouled to the point the entire detector needs to be sent out for refurbishing. Partially plugged FID jets need to be replaced. PID windows can become layered with contamination and require cleaning.
The more instrument downtime you experience, the fewer billable samples you will be able to run in a given period of time. When you have a backlog of analyses to perform, you simply cannot afford unnecessary instrument downtime.
Conclusions
Low detection limits, positive mass spectral identifications, and instruments that are not down for maintenance are all important daily goals for a GC analyst. Using a capillary column, such as Supelco SLB-5ms, that has a low bleed characteristic is an important step in achieving these goals.
Special Purpose SLB-5ms Capillary GC Columns
Phase: Bonded and highly crosslinked; silphenylene polymer virtually equivalent in polarity to 5% phenyl polymethylsiloxane Temp. Limits: 0.20 to 0.32 mm I.D. columns: -60 °C to 340 °C (isothermal) / 360 °C (programmable)
Extend Column Life With Guard Columns
A decrease in peak shape quality in a capillary GC system can typically be traced to the inlet end of the column. Over time, the inlet end of the column becomes contaminated from an accumulation of non-volatile material. The phase can also be damaged from the continuous condensation and vaporisation of solvent and analytes. Inevitably, active analytes will adsorb to the contaminated / damaged section, leading to peak tailing, loss in resolution, and reduced response. When chromatography degrades to an unacceptable level, performance is restored by clipping the contaminated / damaged section off the inlet end of the column.
To extend the lifetime of capillary GC columns, Supelco recommends using a 3 m long guard column. A guard column is a short piece of uncoated deactivated fused silica tubing which is placed in-line between the GC injection port and the analytical column. The guard column will take the brunt of the contamination / damage. By clipping the guard column periodically to restore performance instead of the analytical column, the analytical column remains unaltered. Therefore, chromatography (retention times and resolution) is not affected.
Fused Silica Guard Columns
For use as guard columns to protect your analytical column from damaging sample components. Match the deactivation of the tubing with the polarity of the injection solvent.
Capillary Column Butt Connector
This device consists of a double-tapered ferrule and a stainless steel compression housing with a threaded cap. Small and light (2.3 cm x 0.6 cm, 4.4 g with ferrule), it provides a gas tight seal. This unit maintains inertness with no change in column efficiency
For a complete listing of Supelco Low Bleed SLB-5ms capillary columns, visit our web site sigma-aldrich.com/capillary-ms.
To continue reading please sign in or create an account.
Don't Have An Account?