Ion Chromatography & Cation Suppression
Straightforward Cation Analysis at Trace Levels
Introduction
Trace analysis of cations, amines, and transition metals by ion chromatography can be carried out with or without ion suppression. However, some applications require particularly a highly sensitive analysis. This can only be achieved by sequential suppression, as suppression considerably lowers the detection limits of the analytes.1 Such analyses are common, for instance, in power plant or pharmaceutical applications. Moreover, there are several norms and standards that request for a suppressed cation analysis (e.g. the ASTM D6919 - 17 Standard Test Method for Determination of Dissolved Alkali and Alkaline Earth Cations and Ammonium in Water and Wastewater by Ion Chromatography). In short, suppression reduces background conductivity to a minimum and decreases baseline noise. Both effects together improve the signal-to-noise ratio and increase the sensitivity of the measuring system. Thus, whenever the quantification of very low concentrations of cations is required, analysis with sequential suppression is the method of choice.
Analyses that benefit with the use of sequential suppression
Some typical examples of cation suppression are:
- Traces and ultratraces of Na in the presence of monoethanolamine at high concentrations (typical of sample matrices in nuclear power plants).
- Trace and ultratrace concentrations of alkali and alkaline earth metals such as Li, Na, K, Mg, or Ca, and NH4+ in ultrapure water.
- Traces of transition metals, e.g. Co, Ni, Zn, Mn, and Cd in various types of water samples.
- Aliphatic and aromatic amines in pharmaceuticals, e.g. piperazine in cetirizine·HCl, tetrabutylammonium in atorvastatin, dimethylamine in meropenem, dimethylamine in imatinib mesylate, and meglumine in meglumine salts.
How does sequential cation suppression work?
The term "sequential suppression" represents the combination of chemical suppression immediately followed by CO2 suppression.
Chemical Suppression
The Metrohm Suppressor Module (MSM) is conditioned/ regenerated using a carbonate buffer (78698). The anion exchange resin causes the conversion of all counterions into their respective hydrogen carbonate salts. Dissociated acids (e.g. nitric acid) are used as the eluent. In addition, trace amount of rubidium is added to stabilize the baseline in trace analysis (78737).
The eluent counterions are also replaced with hydrogen carbonate. The carbonic acid produced in this way is unstable and only weakly dissociated, resulting in the measurement of a lower background conductivity, in contrast with the non-suppressed eluent.
Sequential Suppression
For sequential suppression, chemical suppression is combined with subsequent CO2 suppression. This is accomplished with a Metrohm CO2 Suppressor (MCS). In the MCS, the eluent is passed through a capillary made of a gas-permeable membrane surrounded by a vacuum. This removes the carbon dioxide formed, and hence all the hydrogen carbonate from the flow path. All what remains in addition to the analyte is mainly water.
The sequential suppression configuration described above reduces background conductivity (< 0.2 μS/cm) and increases the detection sensitivity of the analytes. Suppression makes the injection peak very small. This means higher resolution between the injection peak and the early eluting cations, e.g. lithium, which makes the integration and quantification of these peaks easier.
Determination of ammonium in acidic absorption solution2
Ammonia scrubber systems are required by law to be used by manufacturers in the chemical industry to reduce or eliminate their ammonia gas emissions. The employed acidic scrubber solutions typically have a pH of 2 or lower. This pH value is too low for silica-based IC columns generally applied in direct conductivity detection of cations. The Metrosep C Supp 2 is polymer-based and allows the injection of low pH samples. As a proof of concept, in this work two acidified drinking water samples spiked with 0.15 and 0.4 mg/L of ammonium were analyzed. The results as shown in Figure 1 and Table 1, indicate that the analysis of such acidic solutions by conductivity measurements can be carried out after sequential cation suppression.
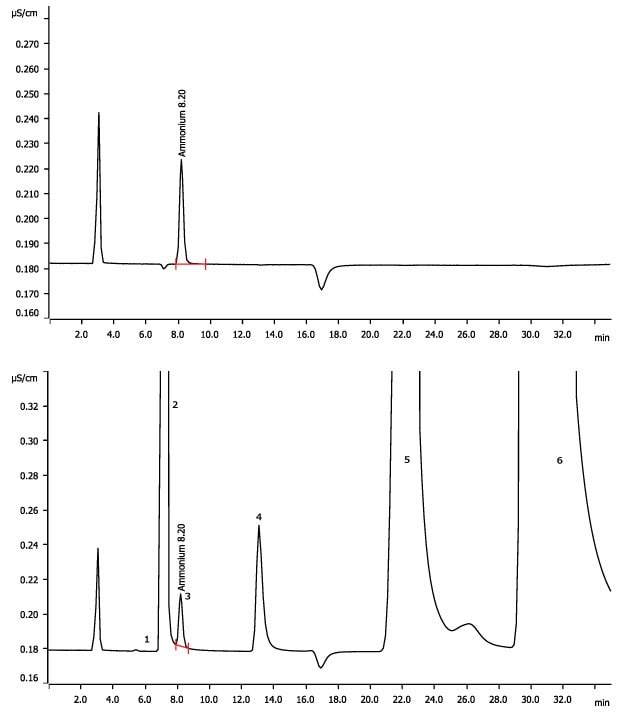
Figure 1.Chromatogram of an acidified standard (top) and acidified drinking water sample spiked with ammonium (bottom). Peak 3 is NH4+.
The measured ammonia concentrations (Peak 3 in Figure 1) are given in Table 1. Li+(1), Na+(2), K+(4), Mg2+(5), Ca2+(6) were not quantified. The peak between Mg and Ca might be Zn. Negative peak at 17 min corresponds to the Rb in the eluent.
Conditions
Conclusion
This article demonstrates that the acidic binary IC eluent nitric acid/rubidium nitrate concentrate and the sodium bicarbonate/sodium carbonate suppressor regenerant for use with Metrosep C Supp 2 column, allows for the accurate determination of ammonium in acidic solutions, such as those found in ammonia scrubbers. We also offer a representative range of inorganic cation standards as certified reference materials (CRMs) for IC.
For a comprehensive overview of our product range for ion chromatography, please visit SigmaAldrich.com/ic
Related Products
References
To continue reading please sign in or create an account.
Don't Have An Account?