Ultrapure Water for LC-MS Biomedical Analyses
The suitability of reagent water used in liquid chromatography coupled with mass spectrometry (LC-MS) biomedical analyses was evaluated and the role of water quality in achieving sensitive and reliable LC-MS analyses is discussed.
Applications of LC-MS in Biomedical Research
The power of LC-MS is recognized by biomedical laboratories worldwide.1,2 Both research and clinical laboratories use LC-MS instrumentation, which is useful in such areas as:
- Therapeutic drug monitoring – measure of therapeutic drugs (e.g., immunosuppressants) in plasma, blood or tissues
- Drugs of abuse testing – measure of illegal drugs (e.g., cannabis, methadone, amphetamine, morphine, meperidine, etc.) in urine or saliva
- Hormone testing – measure of hormones (e.g., steroids or thyroid hormones) in serum or plasma
- Biogenic amine analysis – measure of biogenic amines (e.g., catecholamines) in plasma or urine
- Neonatal screening – monitoring of amino acid and acylcarnitine levels in infants to detect treatable disorders
LC-MS instrumentation is an attractive option over other analytical tools because of the technique's ability to measure multiple complex analytes simultaneously with a very high level of sensitivity. LC-MS biomedical analysis is a rapid process that provides a high level of sensitivity, traceability and reliability. This speed and confidence in results are critical pillars of patient care.
Impact of Water Quality on LC-MS Results
Water contaminants can severely affect the quality of mass chromatograms and mass spectra, leading to unreliable results.
- Particles and Colloids: These can interfere with instrument operation by damaging the (U)HPLC pump and injector. They may also plug the column and frits, increasing backpressure. Colloids can irreversibly adsorb on the stationary phase, reducing the separation efficiency of the column.
- Inorganic ions: Ionic contamination can be an issue when ions form adducts, creating additional peaks in mass spectra, complicating data analysis, and creating interferences with measured analytes.
- Organics: Organic molecules can accumulate on chromatography column beads, reducing resolution and contributing to reduced column lifetime. They may compete with sample molecules, lowering sensitivity and affecting elution efficiency. They may also create ghost peaks and interferences, and cause baseline drifting,
- Bacteria and their by-products: Bacteria can clog columns and frits, increasing backpressure. Bacteria can also release organic by-products to create ghost peaks. (See other effects of organic contamination above.)
Role of ultrapure water in LC-MS biomedical applications
The role of ultrapure water for the biomedical LC-MS workflow and its role in successful LC-MS analytical practice are examined according to three major requirements: sensitivity, traceability, and reliability.
LC-MS Method Sensitivity
Ultrapure water is used extensively in the LC-MS workflow (Figure 1) and thus can become a main source of contamination resulting in ghost peaks, noisy baselines and high MS background. These, in turn, lead to poor instrument or method sensitivity and make it challenging to detect analytes at low concentration.3 Moreover, to avoid interferences, it is essential that the detected analytes come from the samples, and not from the water used in various steps of the experiment.4 Therefore, ultrapure water of very high quality must be used in LC-MS experiments and handled carefully to avoid its recontamination.5
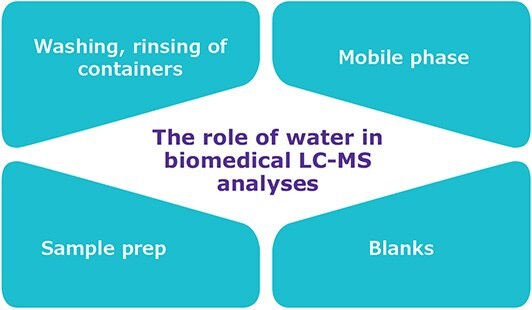
Figure 1.The role of ultrapure water in the LC-MS laboratory
When performing ultratrace LC-MS biomedical analysis of hormones, water is used in significant volumes compared to other mixture components. Thus, ultrapure water from a Milli-Q® water purification system (resistivity 18.2 MΩ.cm, TOC < 5 ppb), used in the sensitive LC-MS analysis workflow, was analyzed for estradiol, an example of a frequently measured hormone in biomedical labs.
The ultrapure water tested was produced in 2 steps: first, a Milli-Q® pretreatment system similar to the Milli-Q® IX Pure Water system and comprising intelligent reverse osmosis, Elix® electrodeionization, and a bactericidal UV lamp delivered pure water; second, this water was further purified to ultrapure water by a Milli-Q® polishing system similar to the Milli-Q® IQ 7000 Ultrapure Water system.
Figure 2 presents an MRM (multiple reaction monitoring) chromatogram that demonstrates the absence of estradiol in Milli-Q® ultrapure water. The lack of estradiol contamination in the reagent water used in the experimental protocol helped to achieve a low method detection limit. As a result, estradiol was successfully detected in a sample containing 265.40 ng/L of estradiol by the standard addition method.
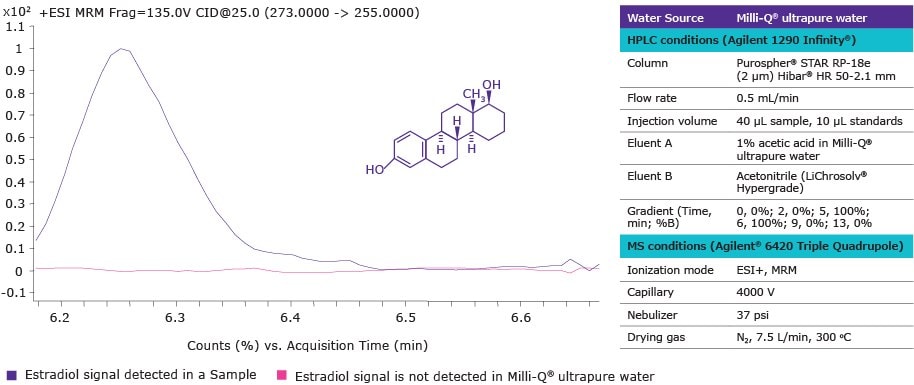
Figure 2.MRM chromatogram (ESI+) of estradiol in a sample and in Milli-Q® ultrapure water. Precursor ion 273 m/z and fragment ion 255 m/z were used for multiple reaction monitoring (MRM) ESI+ transition.
Traceability of Water Quality
Online monitoring functionalities of the water purification source enable scientists to be sure that the water they collect for use in analysis conforms to LC-MS requirements. Some Milli-Q® water systems automatically save system and water quality data and provide simplified quality monitoring, data searchability, and traceability. In the situation where issues generated by contamination have already appeared during LC-MS analyses, it is critical to find and eliminate the source of impurity before repeating experiments, as there are many other contamination pitfalls.6,7 Access to the electronic data that display water quality parameters recorded at the time of water collection for LC-MS experiments can facilitate water evaluation by linking it to the source of its contamination on a particular day of analyses. Moreover, in any clinical laboratory, traceability is a critical demand often linked to quality management systems that allow laboratories to seek accreditation, for example, to the ISO 15189:2007 standard or CLSI® C3 A4. In this situation, an electronic solution for water quality parameter recording is a practical solution that ensures a high level of certainty.
Reliable LC-MS Results
To fulfill the major requirements of LC-MS in the biomedical laboratory, the water source must be reliable. That is, the water purification system must not only produce water of high quality, but this quality must be consistent from one day to another. Online monitoring tools ensure the consistency of water quality. The ionic purity of water is assessed by measuring resistivity, where a resistivity of 18.2 MΩ.cm refers to water free of ionic contaminants. To determine organic contamination, Total Oxidizable Carbon (TOC) is measured; water with TOC below 5 ppb (or μg/L) is suitable for LC-MS practice.
To examine the consistency of Milli-Q® ultrapure water quality, continuous measurements of resistivity and TOC parameters were performed. Results displayed in Figure 3 demonstrate stability in water quality delivered from a Milli-Q® ultrapure system that was monitored continuously online.
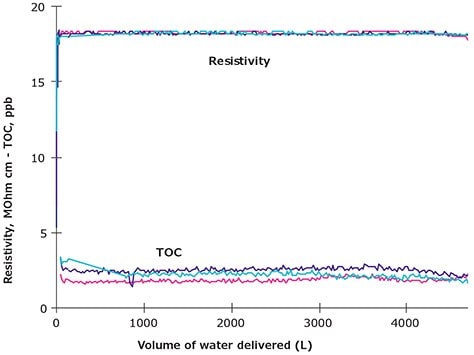
Figure 3. Levels of Resistivity (MΩ.cm) measured continuously and TOC (ppb) measured as a function of volume produced by a Milli-Q® ultrapure water system. Different colors refer to data obtained for three different sets of consumables installed in turns.
Suitability of Milli-Q® ultrapure water for biomedical LC-MS analyses
Modern LC-MS techniques depend heavily on ultrapure water to minimize impurities in the preparation of samples, standards, blanks, and mobile phases. The use of ultrapure water significantly reduces the risk of contamination, which can lead to ghost peaks and inaccurate data interpretation. This reliability is paramount in biomedical applications, where precision is critical for patient care and research outcomes.
This study evaluated the suitability of ultrapure water in meeting critical needs for sensitivity, traceability, and reliability in LC-MS biomedical analyses. It demonstrated the necessity of using water that is of high and consistent quality. Clinical laboratories using LC-MS can depend on Milli-Q® ultrapure water purification systems to achieve both a high level of LC-MS instrumentation sensitivity, and a reliable and traceable analytical process.
A range of water purification solutions are available to meet the needs of laboratories performing LC-MS analyses.
Related Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?