Stem Cell Testing of PES Membrane Containing Stericup® Quick Release Filters
Summary
Though microporous filtration is the method of choice to prepare and minimize contamination of cell culture media and reagents, there still remains some concern that the filtration step will remove valuable factors and/or add deleterious components to the growth media. This concern is especially true for stem cell culture where factors can be expensive, the cells can be very sensitive to additives, and culture durations may be very long. Here we show that a PES membrane containing Stericup® Quick Release filter, even after many multiple filtration steps, provides a worry-free alternative for the pluripotent expansion of embryonic stem cells.
Background
Maintaining sterility is vital during the culture of cells; therefore, great care is taken to prevent contamination. Several methods have been routinely used for the sterilization of liquid reagents, including autoclaving and microporous filtration. Autoclaving has many limitations, in that it cannot be used with liquid growth media containing heat labile ingredients such as proteins and other growth factors, and it is time consuming for other more basic buffers. For these reasons, filtration is the method of choice for sterilization of media components and reagents for cell culture.
Filtration through a membrane with 0.2 μm or smaller pore size allows for the efficient removal of contaminates including bacteria, mold and yeast. There are several filter materials typically used in the sterilization of liquid components including nylon, polycarbonate, cellulose acetate, polyvinylidene fluoride (PVDF, Durapore® membrane), and polyethersulfone (PES). These filter materials differ greatly in their protein retention, flow speed, and the presence of leachable materials. PVDF and PES are both very low protein binding materials without leachables that have wide applications in filtering biological liquids. PES filters are generally preferred for media filtration due to their faster flow rate, especially with more viscous, serum-containing media.
As cell media becomes more defined with expensive factors and cells are cultured for longer and longer durations, the need for filtration to assure sterility and retain essential factors is more important than ever. In addition, filtration must not leach any materials into the media that may be toxic or cause other deleterious effects. This is particularly true with stem cell cultures that are very sensitive to the concentrations of growth factors in the media for both long term pluripotent maintenance and differentiation procedures. For these reasons, it is common practice to add these factors after the sterile filtration of the media due to the fear of removing these factors. Here we demonstrate that the loss of important factors due to filtration is minor and that filtration — even multiple filtrations — does not have any deleterious effects on the growth media.
This study investigated the retention of growth factors required for the expansion of pluripotent embryonic stem cells after multiple sterile filtrations. Although media is typically only filtered once before use, we show that the Stericup® Quick Release filters can be used to filter media multiple times (up to ten times sequentially through new filters) without removal of important factors or the addition of deleterious materials. This observation is shown quantitatively by examining the retention of Leukemia Inhibitory Factor (LIF, a factor required for the maintenance of pluripotency of murine embryonic stem cells, or mESCs) as a function of multiple filtrations through a PES membrane Stericup® Quick Release filter device. In addition, the ability to maintain proper expansion of mESCs for up to five passages in multiply filtered media is shown by appropriate colony morphology of low density colonies and immunohistochemical analysis for markers of plurpotency.
Results
Leukemia Inhibitory Factor (ESGRO®, mLIF medium supplement) has been shown to be a critical media additive for maintaining mouse embryonic stem cells in an undifferentiated state when feeder layers of mouse embryonic fibroblasts are not used. We tested the LIF retention of Stericup® Quick Release filtration devices with PES membranes during filter sterilization.
ESGRO® media supplement containing media was filtered through multiple new PES Stericup® Quick Release filters. Media samples from each filtration were saved for both retention testing and the ability to maintain pluripotent expansion. Up to ten filtrations through separate PES Stericup® Quick Release filters allowed for a ‘worst case scenario’, even though 10 filtrations are unlikely in general work.
Relative LIF retention was determined through the use of LIF-specific sandwich ELISA assays (R&D Systems) as per kit instructions within a linear range of LIF concentration response. All samples were tested in duplicate using unfiltered media as the 100% control. A negative control of media lacking LIF was additionally included for background determination. Sample concentrations were determined by comparison with the standard curve. For 3 lots of filters, Results (Figure 1) show that roughly 90% of LIF is retained after ten filtration steps through PES Stericup® Quick Release filters at a rate of loss of approximately 1% per filtration. This loss allows for acceptable levels of LIF to remain present in the media to assure stem cell pluripotency.
Functional testing of the samples was performed to assure that multiple filtration did not affect the activity of LIF or add deleterious leached components into the media. 500 mL stocks of media were filtered once each, or were filtered 10 times through successive new PES filters. Three lots of PES Stericup® Quick Release filters were tested per filtration point for a total of 6 stocks. Each stock of media was then used for feeder-free maintenance of mESCs for a total of 5 passages on gelatin coated plastic tissue culture plates. After five passages, mESC colonies were observed for pluripotency by alkaline phosphatase staining and Oct-4 (a transcription factor marker for pluripotency) immunohistochemical analysis. Cultures expanded and passaged at low density in all media samples tested (different PES-Stericup® Quick Release filter lots with one or ten filtrations) show colony morphology indicative of pluripotent expansion as well as express appropriate levels of the pluripotency markers alkaline phosphatase (Figure 2) and Oct-4 (Figure 3)
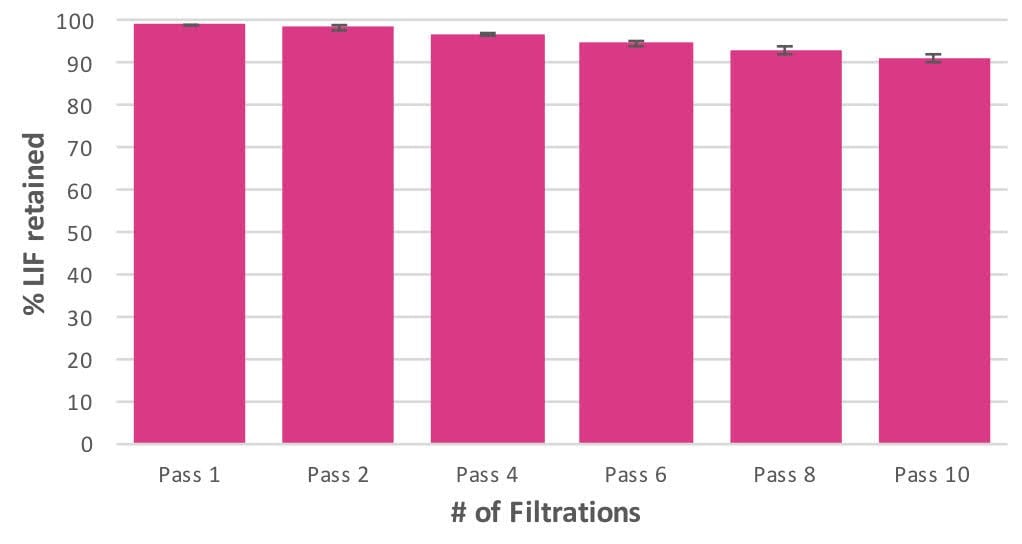
Figure 1. Leukemia Inhibitory Factor (LIF) retention as a function of multiple sequential filtrations through new PES-membrane Stericup® Quick Release Filtration units.LIF concentration in ESGRO® supplement containing treated media was determined by sandwich ELISA assays within a predetermined linear range of response. Percentages are normalized to unfiltered media as 100%.
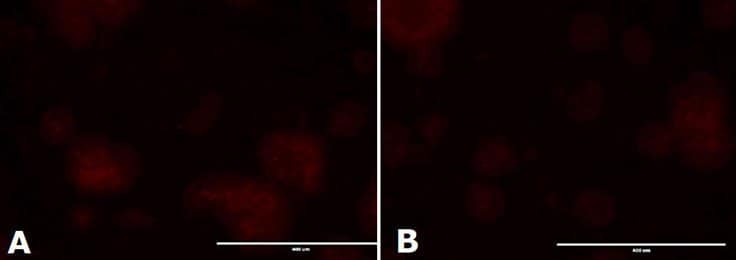
Figure 2.Alkaline Phosphatase staining of mESC colonies. mESC colonies were cultured on 0.1% gelatin coated solid bottom plastic plates with media filtered through PES-Stericup® Quick Release filters once (a) or 5 times (b). Colonies were fixed with 3.7% formaldehyde in PBS for 2 minutes. Napthol/Fast Red Violet solution was added to each well and incubated for 15 minutes before rinsing with TBST buffer.

Figure 3.Immunocytochemistry Analysis of mESC colonies. mESC colonies were cultured on 0.1% gelatin coated solid bottom plastic plates with media filtered once (a) or 5 times (b). Colonies were fixed with 3.7% formaldehyde and permeabilized with 0.1% saponin. Expression of Oct-4 was determined with primary anti-Oct-4 antibody followed by visualization with a Cy3 conjugated secondary antibody.
Materials
To continue reading please sign in or create an account.
Don't Have An Account?