Diethylaminosulfur Trifluoride (DAST)
Because of its ease of handling and versatility, DAST is an extremely popular reagent for nucleophilic fluorination. It has regularly been used in selective fluorinations of alcohols, alkenols, carbohydrates, ketones, sulfides, epoxides, thioethers, and cyanohydrins. Additionally, some novel organic cyclizations are possible when DAST is used as a reagent.1
Fluorodeoxygenation was achieved using DAST in a preparatively simple synthesis of 5,5-difluoropipecolic acid from glutamic acid (Scheme 5).2
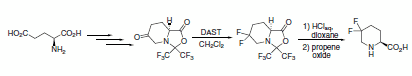
Scheme 5
1,2,2-Trifluorostyrenes can be synthesized using a sequential reaction on the parent a-(trifluoromethyl)phenylethanol with DAST, followed by dehydrohalogenation with lithium bis(trimethylsilyl)amide (LHMDS). This method achieves the trifluorostyrene without requirement of palladium coupling (Scheme 6).3
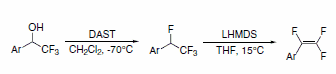
Scheme 6
DAST was used to obtain fluorinated analogues of 3,6-dibromocarbazole piperazine derivatives of 2-propanol (Scheme 7). A series of these analogues are described as the first small and potent modulators of the cytochrome c release triggered by Bid-induced Bax activation in a mitochondrial assay.4
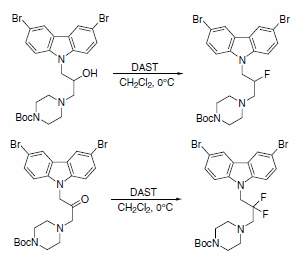
Scheme 7
The synthesis of a,a-difluoroamides via direct fluorination was reported using DAST as the fluorinating reagent in a one-pot reaction (Scheme 8). Decreasing the molar ratio of DAST to substrate resulted in the formation of the respective a-ketoamide.5
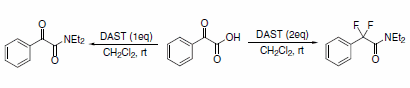
Scheme 8
a-Fluorosulfides and secondary alcohols were coupled by Yb(OTf)3 to generate O,S-acetals, which are key intermediates in the assembly of ciguatoxins. In their synthesis, hydrogen was directly converted to fluorine using DAST and a catalytic amount of SbCl3 to make the a-fluorosulfide (Scheme 9).6
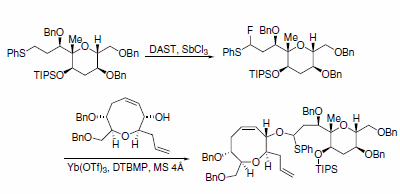
Scheme 9
References
To continue reading please sign in or create an account.
Don't Have An Account?