Ketosulfoxonium Ylides
Introduction to Ketosulfoxonium Ylide Chemistry
Since Corey and Chaykovsky first investigated ketosulfoxonium ylides in the 1960s,1 these reagents have become increasingly popular as stable and safe α-diazocarbonyl equivalents, including for large scale reactions.2-4 Baldwin initially reported on the transition metal-catalyzed reaction of ketosulfoxonium ylides as carbene equivalents for insertions into heteroatom bonds.5 Merck and Co. further advanced this approach,6 including for manufacturing processes,3,4 which resulted in an increased interest in these reagents. In 2017, the labs of Li7 and Aïssa8 first applied ketosulfoxonium ylides to transition metal-catalyzed C-H functionalization, eliciting a rapid upsurge in the number of applications to give diverse products.9 For example, these reagents have been applied to efficient functional group compatible annulations to access privileged [5,6]-bicyclic heterocycles with ring-junction nitrogens, including azolopyridines10 and azolopyrimidines.11,12
The first ten ketosulfoxonium ylides below allow researchers to carry out an initial assessment of the scope and limitations of these reagents for new reaction development and potential drug discovery applications.
Advantages of Ketosulfoxonium Ylides
Ketosulfoxonium ylides are readily prepared in one step from the corresponding carboxylic acids and their derivatives. These reagents are well-behaved and are typically crystalline solids. They serve as versatile and convenient carbene equivalents that release dimethyl sulfoxide as a byproduct. In contrast, while the corresponding α-diazocarbonyl compounds are certainly very useful reagents, the high energy release of nitrogen gas can cause safety issues.
Representative Metal-Catalyzed Reaction Products
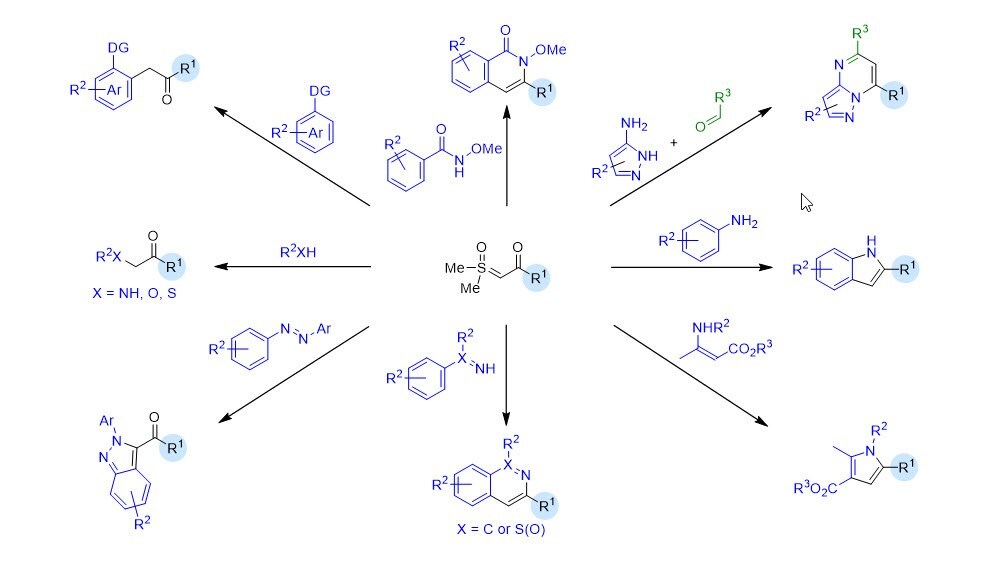
Special thanks to Andrew Streit, Gia Hoang and Jonathan Ellman for contributing this Technology Spotlight!
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?