Validation of a Simple Method for the Alcohol Content in Kombucha Tea by Headspace SPME and GC-MS
Abstract
An assortment of different Kombucha teas was analyzed for ethanol content using an SPME method developed and validated specifically for this application. It was found that many had ethanol levels >0.5%, which is the limit for a beverage to be sold as non- alcoholic in the United States.
Introduction
Kombucha is a fermented tea beverage. It is produced by the addition of a mixture of yeast and acetic acid bacteria, sometimes referred to as “tea fungus”, to a solution of sugar and tea. After fermentation, the result is an effervescent solution containing phenolics, water soluble vitamins, organic acids, as well as some alcohol (ethanol). The health benefits often associated with Kombucha stem from the antioxidant activity of many of these compounds.1,2
In order for Kombucha to be sold as a non-alcoholic beverage in the United States, the alcohol content must be <0.5% by volume.3 Headspace gas chromatography (HS-GC) is a technique that is readily amenable to the analysis of ethanol in a variety of aqueous-based matrices. In the case of Kombucha tea, which can contain a variety of sweeteners and flavoring ingredients, headspace can be used to isolate the volatile alcohol from other constituents in the sample, thus, protecting the GC system. Conventional headspace analysis often requires instrumentation additional to the GC. Headspace solid phase microextraction (HS-SPME) is an alternative approach to the analysis of alcohol content in beverages such as Kombucha. This technique can be performed manually or by automation, does not require the use of a separate concentrator or headspace analyzer external to the GC, and is typically faster and less expensive to perform than other more traditional approaches.
In this work, we have developed an HS-SPME method for the determination of alcohol content in Kombucha. GC-MS was used to allow for accurate and confirmative determination. The HS-SPME method developed is quick, simple, accurate, highly sensitive, and easy to automate.
Experimental Procedure
The final, optimized HS-SPME/GC-MS method is described in Table 1. Alcohol calibration standards were prepared in deionized water at concentrations of 0.10, 0.40, 0.80, 1.00, 1.50, and 2.00 percent alcohol by volume (% ABV) by direct dilution of aliquots of 200 proof ethanol in 25 mL of water. An internal standard/sample diluent solution was prepared at a concentration of 0.08% ABV by direct dilution of neat ethanol-d6 in a 0.05 M sodium phosphate buffer solution (pH=7) containing 25% sodium chloride. This solution was then used in the dilution of samples prior to SPME. The internal standard/diluent solution was prepared daily and chilled prior to use. All samples and calibration standards were diluted 10:1 prior to SPME by addition of 400 µL of each to 3.6 mL of the chilled internal standard/sample diluent solution.
All Kombucha samples tested were purchased at local grocery stores and kept under refrigeration until analyzed. A ginger flavored Kombucha found to have very low alcohol content was used for the preparation of spikes in the method validation process.
Results and Discussion
Optimization of the HS-SPME Procedure
SPME is a very sensitive technique and is normally applied to test for analytes at very low levels. The targeted analytical range for this method was 0.10 to 2.00% ABV, which is considered to be too concentrated for SPME. Thus, the goal was to develop an SPME method that could be used with high accuracy and reproducibility over this entire range. In order to prevent overloading the fiber and detector, sample dilution and the use of a 10:1 split during desorption of the SPME fiber were necessary. Initially, a Carboxen®/ PDMS fiber was chosen for method optimization.
However, a linear response could not be obtained over the entire analytical range. Figure 1 shows a comparison of absolute response by GC-FID using the Carboxen®/PDMS fiber and a 100 µm PDMS fiber over a range of 0.10 to 1.00% ABV. Linearity within this range was slightly better using the PDMS fiber, as evidenced by the higher correlation coefficient (r2) value. Also, the response curve obtained using the Carboxen®/ PDMS fiber started to show some leveling off between 0.80 and 1.00% ABV, and this was expected to become more pronounced at higher concentrations. Since sufficient sensitivity was obtained using the PDMS fiber, it was chosen for further work. After fiber choice, additional parameters such as extraction temperature, time, sample additives, and sample dilution were evaluated during method development. All were optimized to minimize variability in alcohol response from both water and Kombucha tea samples. With the final method, ethanol response was unaffected by sample matrix. This allowed quantitation of samples to be done against a calibration curve made with ethanol in water.
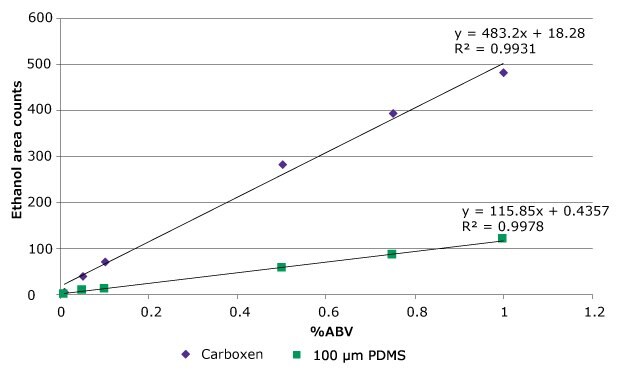
Figure 1.Comparison of alcohol response (GC-FID) from water obtained by HS-SPME; 100 μm PDMS and Carboxen/PDMS fibers.
Method Validation
Accuracy of the HS-SPME GC/MS method was evaluated using ginger flavored Kombucha tea replicates, spiked at concentrations from 0.10 to 2.00% ABV. For the determination of method detection and quantitation limits, replicates spiked at 0.1% ABV were used. Method accuracy, repeatability, detection, and quantitation limits are summarized in Table 2. For the Kombucha spikes, good linearity was obtained, with a linear correlation coefficient of 0.999 from 0.10 to 2.00% ABV. Excellent accuracies of 98-100% ABV were obtained over the analytical range, with method repeatability of <4% RSD. Using the 0.1% ABV Kombucha spikes (n=8), the limit of detection (LOD) and limit of quantitation (LOQ) for the method were calculated as 3x and 10x the standard deviation (0.003) respectively. These calculated values were verified experimentally with the analysis of Kombucha samples spiked at 0.010 and 0.030% ABV. For n=5 spikes at each concentration, accuracy at the LOQ was 95% with a repeatability value of 6% RSD. Accuracy at the LOD was 83% with repeatability of 21% RSD. These low accuracy and poor repeatability values at the LOD can be attributed to the poor response of ethanol at this level, as the signal-to-noise ratio was approximately 2x lower than that obtained at the LOQ, and 4.5x times lower than the 0.1% spiking level. In case of a necessity to quantitate at 0.01% ABV, simple modifications to the method, such as using a splitless SPME injection, could be used to increase the ethanol response.
Analysis of Certified Reference Materials
The method was further validated using certified reference materials of low alcohol beer and pre- prepared solutions of alcohol in water. The results are summarized in Table 3. Each was analyzed multiple times over different days, and in some cases by different analysts on different instruments. The daily accuracies ranged from 96-101% for the beer samples and 94-103% for the alcohol in water solutions with reproducibility of <6% RSD for all sample sets.
Analysis of Kombucha Tea Samples
A total of twenty different Kombucha tea samples from ten different producers (including one homemade sample) were tested using the optimized HS-SPME/GC- MS procedure. All were stored under refrigeration after purchase, and testing was performed on freshly opened bottles before the “best by” date indicated on the label. The results are summarized in Figure 2. All samples in this set were analyzed in replicates of 2 or 3. To determine method reproducibility over multiple days, a subset of eight samples was analyzed in triplicate on two separate days. The samples in this subset varied in alcohol content from 0.39 to 1.66% ABV. Measurement variability was determined as the % RSD in the average value for the 6 replicates of each sample. For all samples, % RSD was < 3%, indicating good repeatability of the method over separate sample batches analyzed on separate days (with all other parameters such as instrument and analyst being the same).
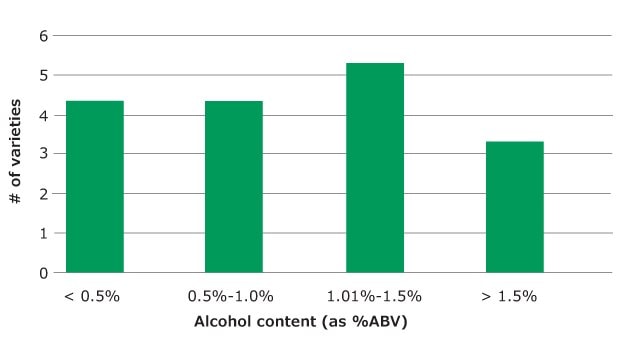
Figure 2.Alcohol (Ethanol) content, in % ABV, measured on 20 Kombucha tea samples using HS-SPME.
Conclusions
An HS-SPME/GC-MS method was developed which can be used to accurately and precisely measure alcohol content in Kombucha tea samples. The optimized method allowed for accurate determination in the range of 0.1 to 2.0% ABV; however, the limit of quantitation indicates that accurate measurement is possible as low as 0.03% ABV. Method repeatability, as demonstrated with analysis of eight different Kombucha varieties, was demonstrated as <4% RSD for replicate measurements made over two days. The dilution approach used in the procedure minimizes matrix effects, thus making it possible to use this HS-SPME method for other low alcohol matrices such as non-alcoholic beer and wine. Applying the HS-SPME/GC-MS method to twenty different varieties of commercially available Kombucha tea samples from nine producers, it was found that most had alcohol levels above 0.5% ABV. This indicates that either the current methodology used for alcohol measurement of these products is not accurate, and/ or fermentation is continuing after bottling, despite refrigeration, and resulting in elevation of alcohol level.
Related Products
References
To continue reading please sign in or create an account.
Don't Have An Account?