Mgat4 May Play a Role in Increased Sialylation by Overexpressing Functional MGAT1 in Mgat1-Disrupted Chinese Hamster Ovary (CHO) Cells
Abstract
MGAT1 adds N-acetylglucosamine to the Man5GlcNAc2 (Man5) structure. Goh et al. reported increased sialylation after restoring MGAT1 function in MGAT1 deficient CHO cells. The hypothesis is that Mgat1 disruption and restoration may lead to altered N-glycan biosynthesis gene expression, which contributes to increased terminal sialylation. Zinc-finger nuclease (ZFN) technology was used to create our Mgat1-disrupted CHO lines with high-mannose dominant simple N-glycoforms. A functional hamster MGAT1 was overexpressed in an Mgat1-disrupted IgG producing cell line (Mgat1 KO37). Five out of sixteen clones isolated from the MGAT1 KO37 OE stable pool demonstrated restored complex N-glycoforms, and two demonstrated 1.4-fold higher sialic acid compared to the non-ZFN transfected line. All Mgat1 KO37 OE clones showed significantly increased Mgat1 expression by q-RT PCR than the endogenous level in the non-ZFN transfected line. The host cell line (CHOZN® GS) had significantly higher endogenous Mgat1 expression than the IgG expressing cell line. Mild increase of the branching enzyme Mgat4 was observed in one of the clones with increased sialylation. The results support the approach of Mgat1 and Mgat4 co-overexpression to create host cell lines for biopharmaceutical production with increased sialylation.
Background and Methods
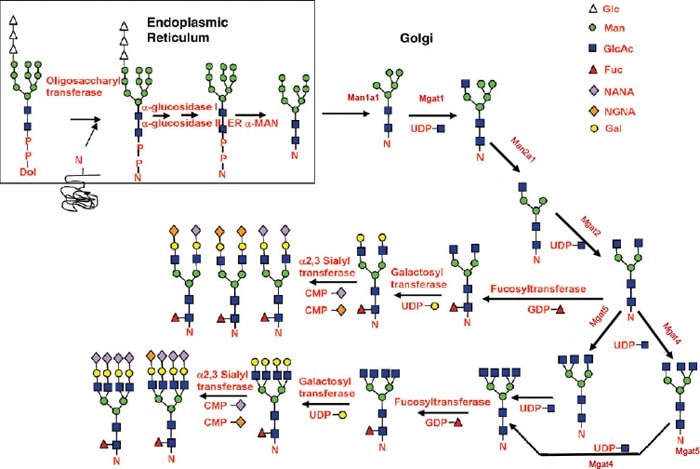
Figure 1.Key Genes in the N-Glycan Biosynthesis Pathway
Literature
- MGAT1 disrupted cells overexpressing recombinant MGAT1 showed increased sialic acid (Goh et al 2010)
- Overexpressing Mgat1 in wildtype CHO cells did not lead to increased sialylation
Figure 2.MGAT1 Overexpression Vector
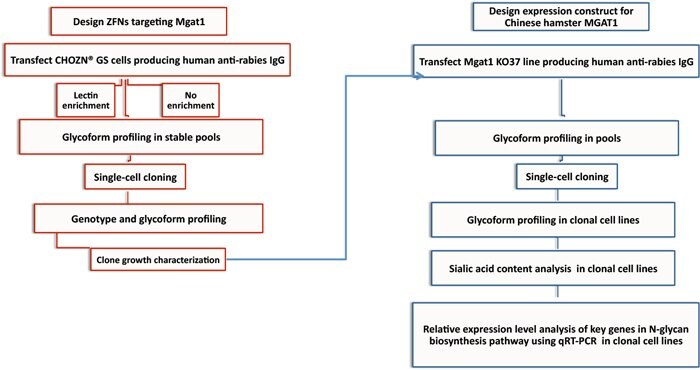
Figure 3.Workflow
All RNA and protein samples were collected from three independent fed-batch growth experiments in TPP Bioreactor tubes (growth data not shown here). Cells were harvested for RNA isolation from Day 4, and for total cellular protein extraction from Day 7. Culture supernatant was collected on Day 7 and purified using Protein A affinity chromatography, and analyzed for sialic acid content using 1,2- diamino-4,5-methylenedioxybenzene (DMB) labeling. Glycoform analysis was performed as described previously (Sealover et al., 2013)
Results
Single-Cell Clones Derived from MGAT1 Overexpression Stable Pool Demonstrate Complex Glycoforms
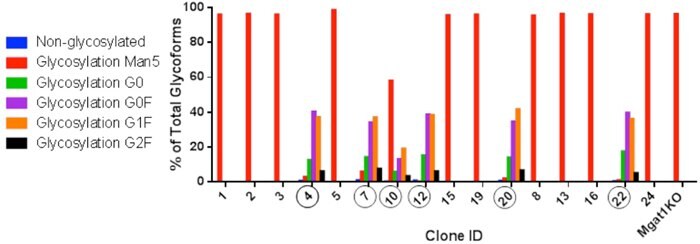
Figure 4.Single Cell Clones
Five out of sixteen clones characterized showed complex glycoforms in purified IgG that these clones produced. One showed some complex glycoforms and high Man5. The other ten clones had similar high Man5 profile as Mgat1KO37. Circled: Zeocin resistant clones that demonstrated restored complex glycoforms.
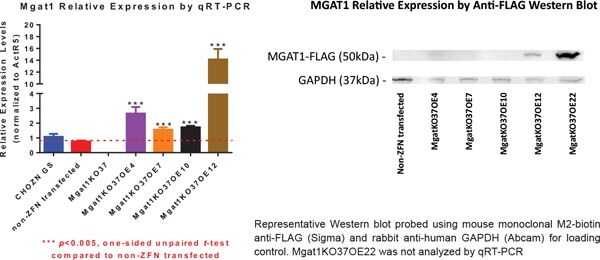
Figure 5.MGAT1KO37 OE Clones Have Higher MGAT1 Expression than the Wildtype Cell Lines
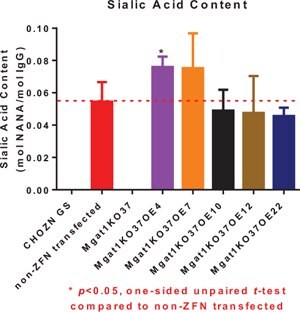
Figure 6.MGAT1KO37_OE4 and OE7 Clones Demonstrate Mildly Increased Sialic Acid Content
Clones OE4 and OE7 showed 1.4 fold increase in sialic acid content. Mgat1KO37 did not have detectable sialic acid. OE 20 was not analyzed for sialic acid content. OE22 was not included in the qRT-PCR experiments.
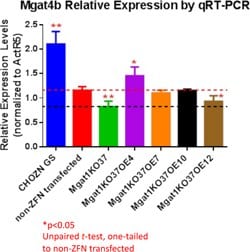
Figure 7.MGAT1KO37_OE4 Have Higher Mgat4b Expression Compared with the Host Cell Lines
All Mgat1KO OE clones have higher Mgat4b than Mgat1KO37.
Mgat1KO37OE4 expresses Mgat4b significantly higher, while OE12’s Mgat4b is significantly lower, than the non-ZFN transfected cell line. OE4 demonstrated the highest Mgat4b expression.
Mgat1KO37 has significantly decreased Mgat4b than non-ZFN transfected cell line where it was derived from.
The host cell line that does not express IgG has significantly higher Mgat4b than the IgG-expressing non-ZFN transfected line.
Mgat2 and Mgat5 did not show any significant change in relative expression (data not shown).
Conclusions
- Disruption and restoration of MGAT1 led to mild increase in sialic acid content in two MGAT1KO OE clones.
- Recombinant MGAT1 expression is essential to restoration of complex glycoforms but may not be the only contributing factor
- All Mgat1KO OE clones demonstrated significantly increased Mgat1 expression
- OE4 and OE7 have similarly elevated sialic acid, but OE4 has higher MGAT1 expression comparing with Mgat1KO37
- The highest MGAT1 expression did not lead to sialic acid increase in OE12 and OE22
- OE10 showed 58% Man5 glycoform despite of its MGAT1 expression similar to OE7 that showed non-detectable Man5
- The branching enzyme MGAT4 may play a role in increased sialic acid
- Disruption of MGAT1 alone without overexpression did not lead to Mgat4b up-regulation.
- After OE some clones exhibited mildly elevated Mgat4b expression
- OE4 has the highest Mgat4b expression and increased sialic acid
- Follow-up studies:
- Glycan structure analysis to confirm that increased sialic acid content is due to branched glycans
- Relative expression of Mgat4a to determine if the isozymes are both up-regulated in clones with increased sialic acid
- Relative expression of CMP-sialic acid transferase, sialyltransferases and sialidases
- Proposed cell engineering strategy: co-overexpression of Mgat1 and Mgat4 to increase sialylation of recombinant therapeutic proteins
Acknowledgements
MilliporeSigma Analytical R&D:
Isil Yasa, Kevin Ray
University of Georgia Complex Carbohydrate Research Center:
Stephanie Archer-Hartmann, Parastoo Azadi
Materials
To continue reading please sign in or create an account.
Don't Have An Account?