Development of a New Advanced KSOM Mouse Embryo Media that Enables Dual-Use Embryo Culture and Cell Handling Applications
Introduction
Transgenic and gene knockout technologies are powerful tools for studying gene function. A popular method for creating transgenic and knockout mice involves the introduction of genetically modified mouse embryonic stem cells into early-stage mouse embryos by either blastocyst injection or aggregation techniques. Culturing mouse embryos in vitro has traditionally been a two-step process of retrieval and cultivation, with each step requiring unique media with characteristics appropriate for harvest or culture conditions. Embryos are commonly removed from the oviducts into a medium buffered with HEPES (M2, FHM). These media maintain their pH in atmospheric, or ambient air. Embryos are then transferred into a second medium which is bicarbonate-buffered (M16, CZB, KSOM) and placed in a culture incubator where the percent CO2 can be precisely controlled to maintain the pH of the medium. Furthermore, it is typically recommended that bicarbonate-buffered media be pre-equilibrated in the incubator for several hours prior to receiving embryos in order to achieve optimal growth.
Our goal was to develop a single medium for both harvest and preimplantation culture of mouse embryos. This media maintains pH in both CO2-controlled and room air conditions, replacing the normal two-media process where one medium is used for embryo harvest and another employed for culture. This novel medium, Embryomax® Advanced KSOM Embryo Media, is based on the original KSOM formulation, with modifications that enable pH buffering in and out of the culture incubator. Along with simplifying the embryo culture workflow, our data indicate that this modified formulation facilitates enhanced embryo development with difficult-to-culture mouse strains, as assessed by the percentage of embryos reaching full blastocyst formation stages.1
Methods
We tested the pH range of FHM, KSOM and Advanced KSOM media under various relevant atmospheric conditions by measuring the pH of each immediately after production, after overnight exposure in the culture incubator at 37°C and 5% CO2, or after overnight exposure on the benchtop. 5 mL of each medium were pipetted into 12 mL Falcon culture tubes, after which the tubes were loosely capped, allowing exposure of the medium to atmospheric conditions. All tests were performed in triplicate.
To assess the in vitro development of one-cell embryos from FVB/N or C57BL/6 mouse strains, we compared embryos that were retrieved in FHM and grown in KSOM to those retrieved and grown in EmbryoMax® Advanced KSOM. We also assessed the effect of pre-equilibration of the growth media by comparing embryos cultured in KSOM or Advanced KSOM with and without pre-equilibration. One-celled zygotes were removed from oviducts 21 hours after hCG administration, and placed into one of four treatment groups:
- KSOM IN - Embryos retrieved in FHM and grown in traditional KSOM (pre-equilibrated)
- KSOM OUT - Embryos retrieved in FHM and grown in traditional KSOM (non-equilibrated)
- KSOMBAL IN - Embryos retrieved in Advanced KSOM (non-equilibrated) and grown in Advanced KSOM (pre-equilibrated)
- KSOMBAL OUT - Embryos retrieved in Advanced KSOM (non-equilibrated) and grown in Advanced KSOM (non-equilibrated)
Embryos were cultured for 72 hours in vitro and scored at 24 hour intervals. At 72 hours, all embryos that had attained more than eight cells were considered normal. Embryos were further classified as morula, cavitating morula (cavity less than half the embryo), or blastocysts (cavity greater than half the embryo).

Figure 1.Percent morula, cavitating morula and blastocysts after 72 hour culture in various mouse embryo media cultivation conditions. KSOM=growth in standard KSOM media. KSOMBAL= retrieval and growth in Advanced KSOM media. IN= growth media was pre-equilibrated. OUT= no pre-equilibration of media.
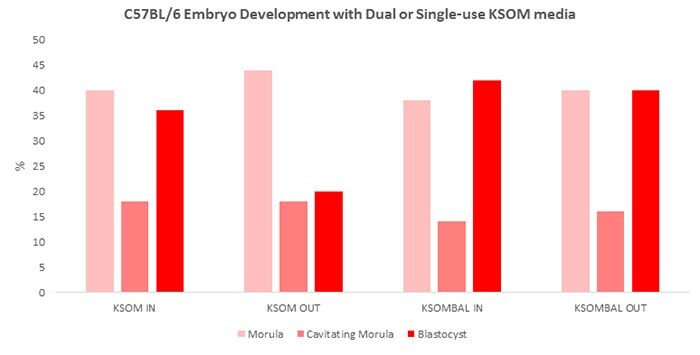
Figure 2.Percent morula, cavitating morula and blastocysts after 72 hours in culture under various mouse embryo media conditions. KSOM=growth in standard KSOM media. KSOMBAL= retrieval and growth in Advanced KSOM media. IN= growth media was pre-equilibrated. OUT= no pre-equilibration of media.
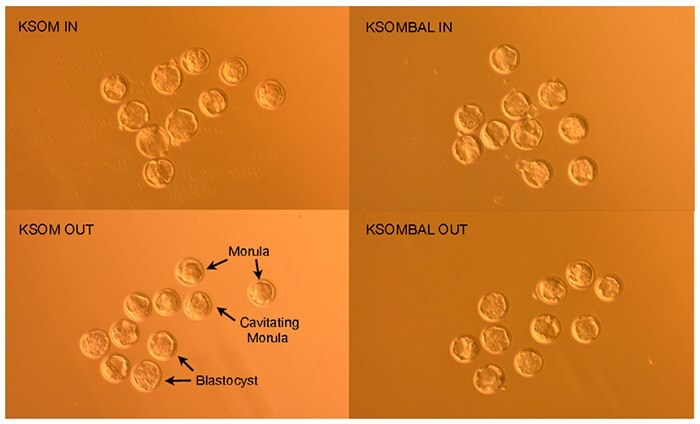
Figure 3.FVB/N embryos 96 hours post-hCG induction of ovulation demonstrate morula, cavitating morula and blastocyst developmental stages.
Conclusions
EmbryoMax Advanced KSOM Medium is a new, uniquely buffered modified version of KSOM that can be used as a single medium to both harvest and culture mouse embryos in preparation for implantation. This medium has been formulated to maintain pH on the bench top or in other ambient conditions, as well as being optimized for controlled CO2 incubation conditions for culturing embryos in vitro. This medium can therefore replace the traditional two-step process where one medium is used for embryo harvest, and a different formulation for culture.
Additionally, our data demonstrate that the Advanced KSOM formulation eliminates the need to pre-equilibrate media before culturing embryos. EmbryoMax Advanced KSOM medium was also shown to produce a higher frequency of blastocyst formation in multiple mouse strains after 72 hours in culture when compared with standard KSOM medium, including difficult to culture strains such as C57BL/6. This medium will simplify the transgenic cell culture workflow and may enhance preimplantation embryo yields for the production of transgenic knockin and knockout mice.
Materials
To continue reading please sign in or create an account.
Don't Have An Account?