Water for PCR Techniques
Polymerase chain reaction (PCR) is the workhorse of modern molecular biology for amplifying specific stretches of DNA or RNA. Since its commercialization in the 1980s, PCR has become essential in diverse research and applied biology fields, including diagnostics, crop science, species preservation, and forensic science. Modern approaches1 include:
- Classic end-point PCR or reverse transcriptase PCR (RT-PCR)
- Multiplex PCR
- Quantitative real time (qPCR)
- Digital PCR (dPCR)
- Crystal digital PCR (cdPCR)
Oligonucleotide amplification occurs through a series of repeated cycles of DNA denaturing, annealing specific oligo primers to the target DNA sequence, and then polymerase binding to the primers and extension of the sequence. As PCR advances, the DNA generated is used as a template for replication. This sets in motion a chain reaction in which the DNA template is exponentially amplified. With PCR it is possible to amplify a single or few copies of a piece of DNA across several orders of magnitude, generating millions or more copies of the DNA piece.
While PCR and related techniques are used to great effect, the process may fail or lead to unexpected reaction products due to DNA, RNA or other genomic contamination.2 In addition, the precision annealing, binding, and replicating steps are particularly sensitive to organic and inorganic impurities in the water used.3,4
Impact of Water Quality on PCR and Related Techniques
The inherent sensitivity and specificity of PCR techniques demands very high purity reagents. PCR sample contamination can significantly affect interpretation with serious downstream consequences. Given the multiple uses of laboratory water in buffers and solutions and for rinsing, using high purity water is critical to prevent contamination and increase reliability and consistency in genomic research.
The water used to prepare samples and buffers used in PCR must be free of the following contaminants:
Nucleases
Nucleases, like DNases and RNases, are fast-acting enzymes that may cleave genomic samples and affect the accuracy and reliability of the PCR amplification process. They may also degrade the target DNA, resulting in false negatives or reduced sensitivity. Due to the sensitive nature of PCR and related PCR-based techniques, nuclease-free water is essential to avoid the degradation of nucleic acids and non-specific amplification of unintended DNA sequences. For the preparation of nuclease-free water, please refer to our technical article, Water for Nuclease-sensitive Molecular Biology Studies.
Ions
Polymerase is sensitive to heavy metals, such as Cd and Zn, as well as a number of divalent metals, in particular transition metals (e.g. Fe, Co, Cu, Ni), that may bind to the site of the enzyme and impact the catalytic activity of the polymerase.3 In addition, a very specific magnesium concentration is required for optimum activity of the polymerase. In order to ensure that no transition metals and magnesium are present in the high purity water selected to prepare buffers, ultrapure water with a resistivity of 18.2 MΩ.cm is highly recommended.
Organics
Negatively charged organic molecules, such as fulvic and humic acids, mimic the DNA charge and may interfere with the catalytic process. They behave as non-competitive inhibitors of the polymerase. Although they do not react with the enzyme, they come in and out of the active site, reducing the turnover of the polymerase. Low TOC value (< 5 ppb or µg/L) is recommended to ensure good water quality in terms of organic content.
Bacteria
Microorganisms are particularly detrimental to PCR based reactions:
- Bacteria liberate nucleases that may cleave the nucleic acids being worked on
- Bacteria contain DNA that can be amplified along with the target DNA
- Bacteria release ions and organics that can interference with the polymerase activity.
Additional Contamination Sources
In addition to the above water contaminant categories, other potential sources of contaminants are diverse (reagents, disposables, sample carry over) and contaminants can be introduced by unrelated activities in neighboring laboratories,4 therefore it is recommended to follow good laboratory practices.
By using a combination of various purification technologies, it is possible to have on-demand ultrapure water adapted to the requirements of PCR techniques.
Effect of Ultrapure Water on RT-PCR Reliability and Reproducibility
Single cell reverse-transcriptase PCR (RT-PCR) was performed on increasing number of pAW109 mRNA copies.5 After amplification, the DNA was separated by electrophoresis and quantified based on densitometry measurement.
A known concentration of an external piece of plasmid mRNA was added. This plasmid RNA was used as an external control. Since the amount of RNA molecules added was known, it was possible to predict the amount of DNA that should result from the amplification of the cDNA, assuming “ideal” conditions.
Figure 1 highlights that the amount of plasmid DNA obtained fits with the expected amounts determined by calculation. The reaction is quantitative. Data shown are an average of 30 experiments. The small standard deviation on those experiments should be noted.
These results are particularly important because working with good-quality water ensures RT-PCR is performed under optimal conditions to obtain the maximum amount of DNA that can be expected.
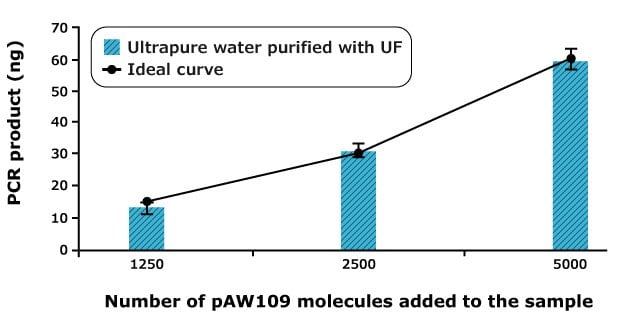
Figure 1.Densitometry graph showing the amount of DNA obtained after RT-PCR on plasmid RNA (pAW109) using ultrapure water from a Milli-Q® system equipped with an ultrafiltration filter. Various concentrations of RNA were added. The ideal points are obtained by the theoretical calculation of doubling the amount of DNA at each cycle.
In Figure 2, the reproducibility of the experiments is demonstrated: 20 experiments were run in parallel, with no significant difference in the DNA amplification.

Figure 2.Reproducibility of plasmid cDNA amplification using ultrapure water with ultrafiltration. The same amount of cDNA was used for each reaction.
The results of these RT-PCR experiments show that when ultrafiltration is used as a final purification stage of the high purity water purification system, quantitative and reproducible results are obtained.
Ultrapure Water for Reliable, Consistent PCR
In conclusion, ultrapure (Type 1) water purified with an ultrafiltration polisher, such as the Biopak® polisher, is highly desirable for PCR and related techniques to avoid contamination and improve repeatability. A range of water purification solutions adapted to the needs of scientists working with DNA and RNA is available.
Select and configure your optimal water purification system for your PCR applications or request support from a lab water solution expert.
Related Products
References
To continue reading please sign in or create an account.
Don't Have An Account?