Intermetallic Hydrides with High Dissociation Pressure
An initial report on the ability of alloys and intermetallics to form compounds with hydrogen dates back to 1958, when Libowitz1 showed that ZrNi easily and reversibly reacts with hydrogen forming ZrNiH3. However, the birth of a new field, chemistry of intermetallic hydrides, is usually considered to be coincident with the discovery a decade later of hydrideforming compounds such as SmCo5 2 and other rare earth AB5 3 and AB2 4 intermetallics.
Practical applications of intermetallic hydrides are based on their chemical interaction with hydrogen. In general, this reaction may be described by the following equation:
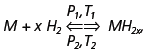
where M is a metal or an intermetallic compound. The rates of reaction for intermetallic compounds differ dramatically from those for individual metals. This peculiarity immediately brought attention to intermetallic hydrides as prospective materials for hydrogen storage and distribution. A majority of conventional materials absorb hydrogen at high rates at room temperature and low pressure. However, practical interest in these intermetallic hydrides is rather limited due to their relatively low reversible gravimetric hydrogen absorption capacity (1.4–1.9 mass.%). At the same time, in light of the greater safety of intermetallic hydrides compared to the hydrides of light metals, and the breadth of experience in applications, the development of new materials with a wide range of operating hydrogen pressures is of great practical interest. Moreover, current availability of novel high-pressure vessels (up to 250–350 atm), and development of 800 atm vessels, facilitate the investigation of high-pressure metalhydrogen systems. Indeed, storing hydrogen in a high-pressure vessel filled with a metal hydride combines the advantages of both compressed gas and solid state hydrogen storage techniques, thus increasing total capacity of the storage container by at least 10%. The metal hydrides based systems with high hydride dissociation/hydrogen desorption pressures are also extremely attractive for applications in high-pressure compressors and internal combustion engines as a part of cold-start ignition systems.
High-Pressure Device
The interaction of intermetallic compounds with hydrogen was studied in a new high-pressure apparatus in the temperature range between 243 and 573 K. A schematic drawing of the high-pressure system is shown in Figure 1.
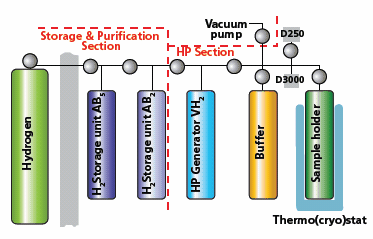
Figure 1. Schematic of a high-pressure (HP) system.
The unit consists of a section for preliminary hydrogen purification and the high-pressure (HP) section. The preliminary purification section consists of a hydrogen source vessel and two hydrogen storage and purification units—one filled with an AB5-type alloy (LaNi5, Aldrich Prod. No. 685933) and the second containing an AB2-type alloy ((Ti,Zr)(V,Mn)2, Aldrich Prod. No. 685941). This configuration prevents possible contamination of the HP generator system, containing vanadium hydride (VH2), by impurities in hydrogen, which may poison the surface of VH2, reduce its absorption capacity and, consequently, hamper its performance. The HP section consists of the HP-generator system containing VH2, a sample holder, a buffer vessel, and two pressure transducers (D250 and D3000) with upper pressure limits of 250 and 3000 atm. Both the sample holder and the HP generator can be heated to high temperatures (up to 573 K) using muffle furnaces. The experimental temperature around the sample holder can also be maintained with a thermostat operating in the temperature range from 243K to 333 K. The data from pressure transducers and from thermocouples attached to the sample holder and the HP generator are collected by a computercontrolled data acquisition system. In order to perform correct hydrogen absorption and desorption calculations, we determined the volumes of all constituent parts of the system. These volumes were obtained in two ways—by calculations based on blueprint dimensions and by volumetric measurements after filling with water. The volume of the sample holder was found to be 8.929 mL. While calculating the total volume of the sample holder, the volume of the alloys and the relevant hydride were also taken into account. A typical sample size during our experiments varied between 15 and 20 g. The amounts of absorbed or desorbed hydrogen were calculated using a modified Van der Waals equation:5
[p+a(p)/Va][V–b(p)] = RT (1)
where, a, b = pressure dependent coefficients (for p>1 atm);
p = pressure (atm);
T = temperature (K);
V = system volume (cm3);
R = universal gas constant (82.06 cm3∙atm/mole∙K).
Thermodynamic parameters of the desorption reaction were determined using the Van’t Hoff equation and fugacity values, corresponding to experimental pressure values:
RT ln(fp) = ∆H–T∆S (2)
where, fp = fugacity;
∆H = enthalpy change;
∆S = entropy change.
Finally, the fugacity values were calculated using Equation 3 and real molar volumes obtained from Equation 1:
RT ln(fp) = RT ln p–p∫0(Vid–Vreal)dp (3)
where, Vreal = real absorbed/desorbed hydrogen molar volume
Vid = ideal absorbed/desorbed hydrogen molar volume
It is also worth noting that our system allows for conducting experimental studies of hydrogen absorption by intermetallics as well as investigating the behavior of various materials at high hydrogen pressures.
YNi5–H2 System
Interaction of hydrogen with AB5-type intermetallics at high pressures has been reported.6 For LaCo5, La0.5Ce0.5Co5, and LaNi5 it was shown that at high hydrogen pressures they form intermetallic hydrides of the approximate composition RT5H9 (R = rare earth metal; T = transition metal), which agrees with theoretical predictions.6 For our studies, we chose a YNi5 alloy because of its unique properties. YNi5 does not easily absorb hydrogen7–9 at low pressures. However, as shown by Takeshita10, applying 1550 atm to the materials allows the synthesis of YNi5H3.5 hydride. Pressure-compositiontemperature (PCT) isotherms obtained led to a conclusion that the pressure applied was not sufficient to obtain a fully hydrogenated sample. The results of other authors11 differed considerably from that of Takeshita.
Our studies showed that an active interaction of YNi5 and hydrogen starts at pressures over 500 atm and the equilibrium hydrogen absorption pressure at 293 K is 674 atm while corresponding equilibrium desorption pressure is 170 atm. Hydride composition at 1887 atm corresponds to YNi5H5 (1.3 mass.% H2). Absorption-desorption PCT-isotherms at the temperatures ranging from –20 to 80 °C are shown in Figure 2. Our data differs from those reported in reference 10, where there are two desorption plateaus at 300 and 1000 atm (293 K) and the hydride composition corresponds to YNi5H3.5. Our data also differs from the results reported in reference 11, where the dissociation pressure is only 12 atm and the hydride composition is YNi5H4.4.
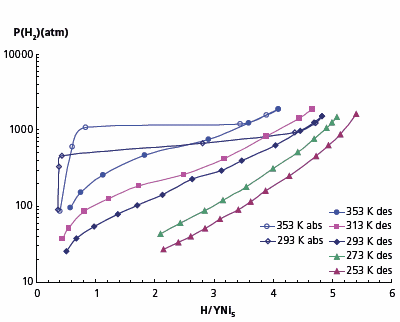
Figure 1. Absorption-desorption isotherms for YNi5–H2 system.
The inconsistency in the numbers reported by different groups may be explained by very low hydrogen absorption and desorption rates observed. In our case, the time to reach equilibrium in the plateau region was between 2 and 4 hours; see Figure 3 which shows the readings of the pressure transducer in several consecutive hydrogen desorption steps.
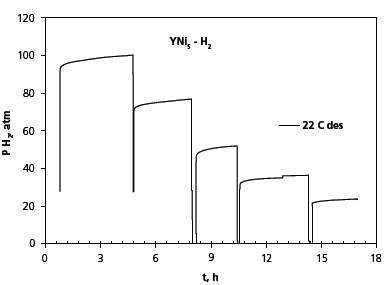
Figure 3. Dependence of pressure change (P) on time (t) to equilibrium.
A number of authors7–9 compared hydrogen absorption properties of YNi5 to other AB5 alloys and concluded that peculiarities in its interaction with hydrogen can be explained neither by the low-temperature heat capacity nor the electronic structure, nor by the surface oxidation of YNi5. In our opinion, the most possible explanation was given in reference 8, where it was shown that among all binary AB5-type intermetallic compounds YNi5 has the lowest compressibility. Thus, the low volume of the YNi5 unit cell could influence hydrogen absorption properties.
Using a lnP(H2) vs. 1/T plot, we found the values of hydrogen desorption enthalpy and entropy of a YNi5 hydride to be 21.86 kJ/mol H2 and 115.8 J/K·mol H2 respectively.
AB2–H2 Systems
As basis materials for these studies, we selected Laves phases ZrFe2 and TiFe2 with high hydrogen desorption pressures, which do not absorb hydrogen at relatively low pressures. It has been reported that using the ultra-high pressure of 10,000 atm, it is possible to synthesise a ZrFe2 hydride.12–14 Our studies showed that noticeable hydrogen absorption in the first absorptiondesorption cycle starts at approximately 800 atm without any preliminary activation. During subsequent cycling, absorption starts at lower pressures. Absorption equilibrium pressure in the first run has been found to be 1120 atm while in second and further cycles it decreased to 690 atm (Figure 4). The hydrogen content in the hydrogenated material at room temperature and 1800 atm is 3.5 H/formula unit. At the low temperature of 218 K and hydrogen pressure of 1900 atm, the material’s composition is ZrFe2H3.7. The isotherms shown in Figure 4 reveal an obvious hysteresis—at room temperature the absorption equilibrium pressure is about 690 atm, while the desorption one is only 325 atm.
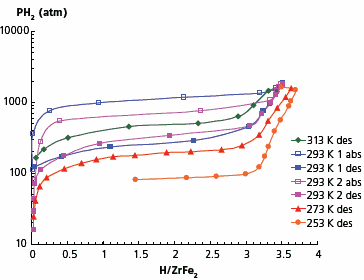
Figure 4. Absorption-desorption isotherms for ZrFe2–H2 system.
Partial substitution of zirconium for scandium reduces the hydrogen desorption pressure of the hydride. Similar to ZrFe2, Zr0.5Sc0.5Fe2 and Zr0.8Sc0.2Fe2 crystallize as C15 Laves phases. Hydrogen absorption in Zr0.5Sc0.5Fe2 starts at ~100 atm without any preliminary activation. There is no significant hysteresis in this system, i.e. the absorption and desorption pressures are very close. Hydrogen content in the material at 295 K and 1560 atm reaches 3.6 H/formula unit (Figure 5).
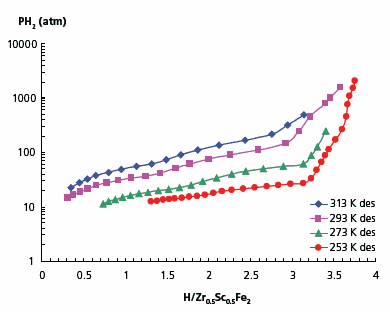
Figure 5. Absorption-desorption isotherms for Zr0.8Sc0.2Fe2–H2 system.
The shape of the hydrogen absorption-desorption isotherms suggests the formation of two hydride phases in the Zr0.5Sc0.5Fe2–H2 system. At room temperature, the composition of the first phase (β1) is close to a dihydride and that of the second one (β2) corresponds to a trihydride (Table 1). Hydrogen desorption enthalpies and entropies have been calculated for both phases. Remarkably, the behavior of Zr0.5Sc0.5Fe2 resembles that of the ScFe1.8–H2 system, where the stable monohydride and the much less stable ScFe1.8H2.4 are also formed.15,16 In our case, however, substitution of half of the scandium for zirconium leads to a significant increase of stability of the lower hydride with an enthalpy of formation lower than that of the trihydride (Table 1).
* Intermetallic compound
** Fugacity
To continue reading please sign in or create an account.
Don't Have An Account?