Tutorial in Nanomaterials
Introduction
Nanotechnology
Nanotools
Nanodevices
Nanostructured Materials
References
Introduction
Although the idea of carrying on manipulations at smaller and smaller scales has been around for quite some time the birth of nanotechnology, at least on an ideological level, is usually traced back to a speech by Richard Feynman at the December 1959 meeting of the American Physical Society. In his speech, Feynman proposed manipulations for such applications as data storage down to the scale of a single atom. It would be over two decades before the first recognized paper on molecular nanotechnology was published.1
Nanotechnology
A motivation in nanoscience is to try to understand how materials behave when sample sizes are close to atomic dimensions. Figure 1 shows a picture of nanofibrils that are 10 to 100 times smaller in diameter than conventional textile fibers. In comparison to a human hair which is ca. 80,000 nm in diameter, the nanofibers are 1,000 times smaller in diameter. When the characteristic length scale of the microstructure is in the 1- 100 nm range, it becomes comparable with the critical length scales of physical phenomena, resulting in the so-called "size and shape effects." This leads to unique properties and the opportunity to use such nanostructured materials in novel applications and devices. Phenomena occurring on this length scale are of interest to physicists, chemists, biologists, electrical and mechanical engineers, and computer scientists, making research in nanotechnology a frontier activity in materials science.
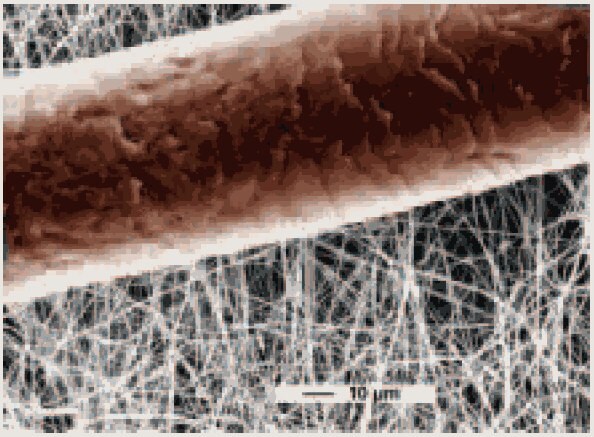
Figure 1. A picture of nanofibrils shown with a human hair for reference (reproduced with permission from Espin Technologies, Inc.)
On tracking the nano evolution, it has been stated that no matter what the market outcomes in the near or long term, nanoscience will never be an industry unto itself but a science of many avenues of application, and possibility that could redefine the direction of several industries.2 This insight allows one to recognize that nanotechnology is not "a technology" but "a set of technologies," yielding a set of technical breakthroughs that will seep into many different markets. Within such a framework, the world of nanotechnology may be divided into three broad categories: nanostructured materials, nanotools, and nanodevices.
Nanotools
Nanotools are devices that manipulate matter at the nano or atomic regime. Devices such as atomic force microscopes, scanning probe microscopes, atomic-layer-deposition devices and nanolithography tools, can manipulate matter in the atomic or molecular regime. Some other nanotools include fabrication techniques; analysis and metrology instruments; and software for nanotechnology research and development. They are used in lithography, chemical vapor deposition (CVD), 3-D printing, and nanofluidics. Nanofluidics, the study of nanoscale fluid behavior, for example, the study of dynamics of droplets adsorbed onto surfaces under shearing43 is mostly used in areas such as medical diagnostics and biosensors.
Nanodevices
Nanodevices are any complete system with nanostructured components that carries out as assigned function other than manipulating nanomatter. The first nanodevices on the market were quantum dot fluorescent biodetectors. MEMS devices are used as accelerometers in automotive airbags. Many other promising applications are in development such as nanoelectric memory devices, nanosensors and drug delivery systems. Components to nanodevices will include nanomaterials, semiconducting organic molecules, polymers and high-purity chemicals and materials.
Nanostructured Materials
In this section:
Nanocrystalline Materials
Carbon Nanotubes/Fullerenes
Dendrimers (Organic Nanoparticles)
Polyhedral Silsesquioxanes (Inorganic-Organic Hybrid Nanoparticles)
Nano-Intermediates
Nanocomposites
Nanostructured (NsM) materials are materials with a microstructure the characteristic length scale of which is on the order of a few (typically 1-100) nanometers. The microstructure refers to the chemical composition, the arrangement of the atoms (the atomic structure), and the size of a solid in one, two, or three dimensions. Effects controlling the properties of nanostructured materials include size effects (where critical length scales of physical phenomena become comparable with the characteristic size of the building blocks of the microstructure), changes of the dimensionality of the system, changes of the atomic structure, and alloying of components (e.g., elements) that are not miscible in the solid and/or the molten state.
The synthesis, characterization and processing of nanostructured materials are part of an emerging and rapidly growing field. Research and developement in this field emphasizes scientific discoveries in the generation of materials with controlled microstructural characteristics, research on their processing into bulk materials with engineered properties and technological functions, and introduction of new device concepts and manufacturing methods.
Nanostructured materials may be grouped under nanoparticles (the building blocks), nano-intermediates, and nanocomposites. They may be in or far away from thermodynamic equilibrium. For example, nanostructured materials consisting of nanometer-sized crystallites of Au or NaCl with different crystallographic orientations and/or chemical compositions vary greatly from their thermodynamic equilibrium. Nanostructured materials synthesized by supramolecular chemistry yielding nanoassemblies are examples of those in thermodynamic equilibrium.
In the paragraphs below, the various classes of nanoparticles that serve as the building blocks of nanomaterials and devices will be discussed. They include nanocrystalline materials such as ceramic, metal and metal oxide nanoparticles; fullerenes, nanotubes and related structures; nanofibers and wires, and precise organic as well as hybrid organic-inorganic nanoarchitechtures such as dendrimers and polyhedral silsesquioxanes, respectively.
Nanocrystalline Materials
Included here are ceramics, metals, and metal oxide nanoparticles. In the last two decades a class of materials with a nanometer-sized microstructure have been synthesized and studied. These materials are assembled from nanometer-sized building blocks, mostly crystallites. The building blocks may differ in their atomic structure, crystallographic orientation, or chemical composition. In cases where the building blocks are crystallites, incoherent or coherent interfaces may be formed between them, depending on the atomic structure, the crystallographic orientation, and the chemical composition of adjacent crystallites. In other words, materials assembled of nanometer-sized building blocks are microstructurally heterogeneous, consisting of the building blocks (e.g. crystallites) and the regions between adjacent building blocks (e.g. grain boundaries). It is this inherently heterogeneous structure on a nanometer scale that is crucial for many of their properties and distinguishes them from glasses, gels, etc. that are microstructurally homogeneous.3
Grain boundaries make up a major portion of the material at nanoscales, and strongly affect properties and processing. The properties of NsM deviate from those of single crystals (or coarsegrained polycrystals) and glasses with the same average chemical composition. This deviation results from the reduced size and dimensionality of the nanometer-sized crystallites as well as from the numerous interfaces between adjacent crystallites. An attempt is made to summarize the basic physical concepts and the microstructural features of equilibrium and non-equilibrium NsM.
Nanocrystallites of bulk inorganic solids have been shown to exhibit size dependent properties, such as lower melting points, higher energy gaps, and nonthermodynamic structures.4,5 In comparison to macro-scale powders, increased ductility has been observed in nanopowders of metal alloys.6,7 In addition, quantum effects from boundary values become significant leading to such phenomena as quantum dots lasers.
One of the primary applications of metals in chemistry is their use as heterogeneous catalysts in a variety of reactions. In general, heterogeneous catalyst activity is surface dependent. Due to their vastly increased surface area over macro-scale materials, nanometals and oxides are ultra-high activity catalysts. They are also used as desirable starting materials for a variety of reactions, especially solid-state routes. Nanometals and oxides are also widely used in the formation of nanocomposites. Aside from their synthetic utility, they have many useful and unique magnetic, electric, and optical properties.8,9
Carbon Nanotubes / Fullerenes
The discovery of fullerenes in 1985 by Curl, Kroto, and Smalley10 culminated in their Nobel Prize in 1996. Fullerenes, or Buckminsterfullerenes, are named after Buckminster Fuller the architect and designer of the geodesic dome and are sometimes called bucky balls. The names derive from the basic shape that defines fullerenes; an elongated sphere of carbon atoms formed by interconnecting six-member rings and twelve isolated five-member rings forming hexagonal and pentagonal faces. The first isolated and characterized fullerene, C60, contains 20 hexagonal faces and 12 pentagonal faces just like a soccer ball and possesses perfect icosahedral symmetry.10
Fullerene chemistry continues to be an exciting field generating many articles with promising new applications every year. Magnetic nanoparticles (nanomagnetic materials) show great potential for high-density magnetic storage media. Recent work has shown that C60 dispersed into ferromagnetic materials such as iron, cobalt, or cobaltiron alloy can form thin films with promising magnetic properties.11,12 A number of organometallic-fullerene compounds have recently been synthesized. Of particular note are a ferrocene-like C60 derivative13 and pair of fullerenes bridged by a rhodium cluster.14 Some fullerene derivatives even exhibit superconducting character.15 There has been a report of a fullerene containing, superconducting field-effect device with a Tc as high as 117 K.16
Carbon nanotubes (CNTs) are hollow cylinders of carbon atoms. Their appearance is that of rolled tubes of graphite such that their walls are hexagonal carbon rings and are often formed in large bundles. The ends of CNTs are domed structures of six-membered rings capped by a five-membered ring. Generally speaking, there are two types of CNTs: single-walled carbon nanotubes (SWNTs) and multi-walled carbon nanotubes (MWNTs). As their names imply, SWNTs consist of a single, cylindrical graphene layer, where as MWNTs consist of multiple graphene layers telescoped about one another.
Carbon nanotubes (CNTs) were first isolated and characterized by Ijima in 1991.17 Since then dozens of research articles have been published, and new applications for CNTs have been proposed every year. The unique physical and chemical properties of CNTs, such as structural rigidity18 and flexibility continue to generate considerable interest. Additionally, CNTs are extremely strong, about 100 times stronger (stress resistant) than steel at one-sixth the weight. CNTs can also act as either conductors or semiconductors depending on their chirality,19-21 possess an intrinsic superconductivity,22 are ideal thermal conductors,23 and can also behave as field emitters.24
Carbon Nanotube-Based Nanodevices
Carbon nanotubes are a hot research area at the moment. The excitement has been fueled by experimental breakthroughs that have led to realistic possibilities of using them commercially. Applications could include field emission-based flat panel displays, novel semiconducting devices, chemical sensors, and ultra-sensitive electromechanical sensors.25
The utility of carbon nanotubes for molecular electronics or computers, first predicted by theory and simulations, is now being explored through experiments to fabricate and conceptualize new devices based on simulations. Carbon nanotubes are now the top candidate to replace silicon when current chip features cannot be made any smaller in 10-15 year’s time. Calculations show that nanotubes can have metallic or variable semiconducting properties with energy gaps ranging from a few meV to a few tenths of an eV. Experiments probing the density of states confirm these predictions. Conductivity measurements on single nanotubes have shown rectification effects for some nanotubes and ohmic conductance for others. These properties suggest that nanotubes could lead to a new generation of electronic devices. Simulations to investigate the interaction of water molecules with a nanotube tip26 revealed an atomistic understanding of the interaction, which is critical in designing commercial-quality flat panel displays around carbon nanotubes. Their use as ultra-sensitive electromechanical sensors has also been explored.
Dendrimers (Organic Nanoparticles)
In recent years, a new structural class of macromolecules, the dendritic polymers, has attracted the attention of the scientific community. These nanometer sized, polymeric systems are hyperbranched materials having compact hydrodynamic volumes in solution and high, surface, functional group content. They may be water-soluble but, because of their compact dimensions, they do not have the usual rheological thickening properties that many polymers have in solution. Dendrimers, the most regular members of the class, are synthesized by step-wise convergent or divergent methods to give distinct stages or generations. Dendrimers are defined by their three components: a central core, an interior dendritic structure (the branches), and an exterior surface (the end groups). Over 50 compositionally different families of these nanoscale macromolecules, with over 200 end-group modifications, have been reported.27 They are characterized by nearly spherical structures, nanometer sizes, large numbers of reactive endgroup functionalities, shielded interior voids, and low systemic toxicity. This unique combination of properties makes them ideal candidates for nanotechnology applications in both biological and materials sciences. The spate of reports in the current literature has been directed toward their applications in a broad range of fields, including materials engineering, industrial, pharmaceutical, and biomedical applications. Specifically, nanoscale catalysts,28,29 novel lithographic materials,30 rheology modifiers,31 and targeted drug delivery systems,32 MRI contrast agents, and bioadhesives represent some of the potential applications.
Polyhedral Silsesquioxanes (Inorganic-Organic Hybrid Nanoparticles)
Hybrid inorganic-organic composites are an emerging class of new materials that hold significant promise. Materials are being designed with the good physical properties of ceramics and the excellent choice of functional group chemical reactivity associated with organic chemistry. New silicon-containing organic polymers, in general, and polysilsesquioxanes, in particular, have generated a great deal of interest because of their potential replacement for and compatibility with currently employed, silicon-based inorganics in the electronics, photonics, and other materials technologies. Hydrolytic condensation of trifunctional silanes yields network polymers or polyhedral clusters having the generic formula (RSiO1.5)n.33,34 Hence they are known by the "not quite on the tip of the tongue" name silsesquioxanes. Each silicon atom is bound to an average of one and a half (sesqui) oxygen atoms and to one hydrocarbon group (ane). Typical functional groups that may be hydrolyzed/condensed include alkoxy- or chlorosilanes, silanols, and silanolates.35
Synthetic methodologies that combine pH control of hydrolysis/condensation kinetics, surfactant-mediated polymer growth, and molecular templating mechanisms have been employed to control molecular scale regularity as well as external morphology in the resulting inorganic/organic hybrids (from transparent nanocomposites, to mesoporous networks, to highly porous and periodic organosilica crystallites) all of which have the silsesquioxane (or RSiO1.5) stoichiometry.36-38 These inorganic-organic hybrids offer a unique set of physical, chemical, and size dependent properties that could not be realized from just ceramics or organic polymers alone. Silsesquioxanes are therefore depicted as bridging the property space between these two component classes of materials. Many of these silsesquioxane hybrid materials also exhibit an enhancement in properties such as solubility, thermal and thermomechanical stability, mechanical toughness, optical transparency, gas permeability, dielectric constant, and fire retardancy, to name just a few.
Nano-Intermediates
Nanostructured films, dispersions, high surface area materials, and supramolecular assemblies are the high utility intermediates to many products with improved properties such as solar cells and batteries, sensors, catalysts, coatings, and drug delivery systems. They have been fabricated using various techniques.
Nanoparticles are obvious building blocks of nanosystems but, require special techniques such as self-assembly to properly align the nanoparticles. Recent developments have lead to air resistant, room temperature systems for nanotemplates with features as small as 67 nm.39 More traditionally, electron-beam systems are used to fabricate devices down to 40 nm.40
Nanocomposites
Nanocomposites are materials with a nanoscale structure that improve the macroscopic properties of products. Typically, nanocomposites are clay, polymer or carbon, or a combination of these materials with nanoparticle building blocks.
Nanocomposites, materials with nanoscale separation of phases can generally be divided into two types: multilayer structures and inorganic/organic composites. Multilayer structures are typically formed by gas phase deposition or from the self-assembly of monolayers. Inorganic/organic composites can be formed by sol-gel techniques, bridging between clusters (as in silsequioxanes), or by coating nanoparticles, in polymer layers for example. Nanocomposites can greatly enhance the properties of materials. For example, ppm level impurities can result in the formation of nanoscale aluminide secondary phases in aluminum alloys, increasing their strength and corrosion resistance. Magnetic multilayered materials are one of the most important aspects of nanocomposites as they have led to significant advances in storage media.24
Polymer-Clay Nanocomposites
The large industrial demand for polymers has lead to an equally large interest in polymer composites to enhance their properties. Clay-polymer nanocomposites are among the most successful nanotechnological materials today. This is because they can simultaneously improve material properties without significant tradeoffs.
Recent efforts have focused upon polymer-layered silica nanocomposites and other polymer/clay composites.41 These materials have improved mechanical properties without the large loading require by traditional particulate fillers.35 Increased mechanical stability in polymer-clay nanocomposites also contributes to an increased heat deflection temperature. These composites have a large reduction gas and liquid permeability and solvent uptake. Traditional polymer composites often have a marked reduction in optical clarity; however, nanoparticles cause little scattering in the optical spectrum and very little UV scattering.42 Although flame retardant additives to polymers typically reduce their mechanical properties, polymer-clay nanocomposites have enhanced barrier and mechanical properties and are less flammable. Compression-injection molding, melt-intercalation, and coextrusion of the polymer with ceramic nanopowders can form nanocomposites. Often no solvent or mechanical shear is needed to promote intercalation.
References
To continue reading please sign in or create an account.
Don't Have An Account?