Click Chemistry in Biomaterials Science
Introduction
Combining biomolecules and synthetic polymers into a new class of versatile biohybrid materials with many (future) fields of application, has gained much interest in recent years. One of the reasons is the current availability of a synthetic toolbox to conjugate biomolecules and synthetic polymers in a controlled fashion. A particularly useful candidate from this toolbox is the copper(I)-catalyzed azidealkyne cycloaddition, or click chemistry methodology, due to the efficiency and specificity of this reaction, along with the possibility of introducing the required functionalities in polymers of both biological and synthetic origin. In this article, an overview is given of the application of click chemistry in macromolecular engineering and the synthesis of polymer biohybrids.
Polymer bioconjugate synthesis
Natural polymers like DNA and proteins possess a level of structural control that is undisputedly superior to current synthetic materials. The well-defined three-dimensional organization stems from a highly controlled arrangement of nucleotides or amino acids, and gives biopolymers their specific functionality. This three-dimensional structure is often responsible for the function associated with a biopolymer, and any conformational changes that can readily occur will result in a loss of function. Synthetic polymers, on the other hand, are lacking this absolute level of control, yet they are easier to prepare in a wide range of distinct topologies and compositions adapted to a specific application environment.
A logical strategy that has surfaced recently is to combine the structural control of biopolymers, leading to properties like programmed assembly, recognition and bioactivity, with the versatility of synthetic polymers. Most of the currently applied biohybrid polymers are synthesized using traditional chemistry, which relies on reactivity towards amine or thiol groups present in lysine and cysteine residues of proteins, respectively. Although impressive results already have been obtained with the biohybrids, these residues, especially lysine, are commonly present in proteins, resulting in multiple additions of the synthetic polymer building blocks. Hence, to prepare well-defined biohybrid polymers, specific coupling chemistry is demanded, which is inert towards other functionalities present in biomolecules.
In this respect, click chemistry is suitable for conjugating biomolecules and synthetic polymers. Click chemistry was termed by Sharpless for a set of chemical reactions that are modular, wide in scope, give high yields, and generate only inoffensive byproducts that can be removed by nonchromatographic methods.1 The most well known example of a click reaction is the copper(I)- catalyzed azide-alkyne cylcoaddition (CuAAC), which yields a 1,4-disubstituted five-membered 1,2,3-triazole ring2, as depicted in Figure 1. This reaction between azides and alkynes offers high yields, and other functionalities do not interfere with the reaction pathway. Both azide and alkyne functionality can be introduced at specific locations in biomolecules and synthetic polymers using currently available techniques. This has allowed click chemistry to revolutionize the fields of polymer chemistry and bioconjugation. Some of these advances will be highlighted in the following sections.

Figure 1. Schematic illustration of the functionalization of side and end groups of polymers using “click” chemistry.
The emerging role of click chemistry in macromolecular engineering
Living/controlled polymerization techniques that are currently available, such as ring-opening metathesis polymerization (ROMP), nitroxide mediated radical polymerization (NMP), reversible addition-fragmentation chain transfer polymerization (RAFT) and atom transfer radical polymerization (ATRP) allow precise control over the polymerization process; the degree of polymerization can be predetermined and the polydispersity is low. Furthermore, tailoring of macromolecular architectures with respect to functionality in the side chains and end groups, composition (e.g. block, graft and gradient copolymers) and topology (e.g. comb, star and dendritic structures) is possible.3 Because azide and alkyne moieties can be readily introduced at different locations in polymer chains by adopting controlled polymerization methodologies, the highly efficient CuAAC reaction has become a powerful tool in macromolecular engineering.4-8
The CuAAC reaction has been applied to modularly construct a myriad of well-defined polymer topologies from azide and alkyne functionalized building blocks, in high yields. In Figure 2, a selection of synthesized architectures is given, ranging from block, graft and star copolymers to polymer networks like hydrogels. For example, by application of ATRP it was demonstrated that azide and alkyne groups can be introduced at polymer chain ends.9 This was achieved by using initiators containing the alkyne functionality, and by post-polymerization substitution with an azide of the halogen, which is always present at the terminus of a polymer prepared via ATRP . This resulted in functionalized polymer building blocks which could be coupled in a quantitative fashion using a copper(I)- catalyst.
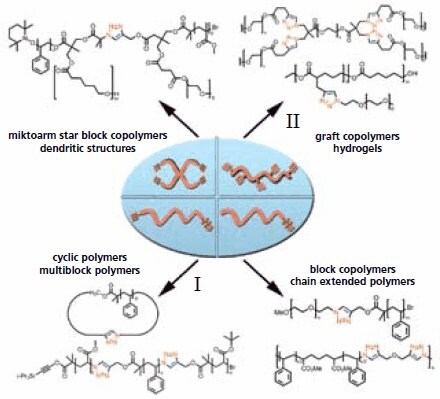
Figure 2. Examples of macromolecular architectures prepared by “click” chemistry: block copolmyers9,10 , chain extended polymers11 , cyclic polymers12 , graft copolymers13 , hydrogels14 and miktoarm star block copolymers15
Because it is also possible to introduce an azide as well as an acetylene end group in the same polymer building block, the click coupling concept was extended to allow consecutive functionalization of both end groups of a single polymer.10 To selectively perform the CuAAC reaction on one end of a polymer, the acetylene moiety at the other terminus was protected with a triisopropylsilyl (TIPS) group that could be easily removed afterwards making it accessible for the second click coupling. Using this modular approach a poly(methyl acrylate)-block-polystyrene-block-poly(tert-butyl acrylate) ABC-type triblock copolymer (Figure 2) was prepared.12 An extension of this modular approach is having blocks prepared by different polymerization mechanisms which are also linked relatively easily. .
Apart from generating a variety of polymer architectures, the CuAAC reaction can also be exploited to introduce functionality along polymer chains and in networks.4-6 Bulky pendant groups can be grafted onto polymer chains by using azide or alkyne functionalized monomers, (reaction II in Figure 2), something that can be problematic when polymerizing pre-functionalized monomers. In general, controlled polymerization techniques and “click” chemistry form a powerful couple with almost unlimited possibilities for the introduction of different functionalities in polymer side chains and end groups.
Biohybrid polymer synthesis via click chemistry
In addition to the fact that the CuAAC reaction is efficient, making it possible to couple large molecules such as polymers, in high yields, the click process is also highly specific. This implies that the used azide and alkyne groups are inert towards other functionalities and merely react with each other in the presence of a copper(I)-catalyst. This specificity combined with the fact that the reaction can be performed in an aqueous environment at ambient temperatures, makes click chemistry an ideal tool for the conjugation of biomolecules, like peptides, proteins, carbohydrates and DNA, to synthetic polymers.16,17 One of the first examples of this approach was the conjugation of an azide terminated polystyrene and the alkynefunctionalized protein bovine serum albumin (BSA),18 as depicted in Figure 3. For this purpose, the azide end functionality was quantitatively introduced by nucleophilic substitution of the halogen terminus of polystyrene, which was prepared by ATRP. The required alkyne function was introduced by Michael addition of an alkyne bearing maleimide to the thiol functionality of a cysteine residue (Cys-34) located on the exterior of BSA, yielding a singly alkynated protein. Subsequently, a click reaction was performed between the synthetic polymer and the protein by addition of cupric sulfate and ascorbic acid, which serves as a reductor to generate the catalytic Cu(I)-species in situ. Interestingly, as a consequence of the amphiphilic character of the isolated biohybrid polymer, micelles were formed in an aqueous environment as established with transmission electron microscopy (Figure 3).

Figure 3. Illustration of the “click” reaction between polystyrene and the protein bovine serum albumin (BSA).18 The resulting amphiphilic biohybrid displayed micelle formation in an aqueous environment as visualized in the transmission electron microscopy image.18
In addition to the bioconjugation of proteins, the CuAAC reaction has also been used to couple carbohydrates, such as mannose and galactose, to linear and dendritic polymers.19,20 These biohybrids, containing multivalent binding sites, can be adopted to interfere in specific pathways in cell-cell recognition and cell-protein interaction processes. Moreover, carbohydrates serve as targeting ligands for hormones, antibodies and toxins, making these materials suitable candidates for use in medicine or as biosensors. Additionally, click reactions have been performed onto more complex biological entities such as viruses, bacteria and cells, clearly underlining the power of this chemistry.
To maintain the function of biomolecules as well as the reproducibility of the biohybrid synthesis, it is of the utmost importance that the necessary alkyne and azide groups be incorporated at desired locations in biomolecules via protein engineering techniques. One approach is the so-called multisite replacement, in which auxotrophic bacterial strains that lack the ability to produce one of the proteinogenic amino acids, are used. If a non-canonical amino acid, e.g. azidohomoalanine, is added to the growth medium, it can be incorporated in place of the natural substrate.21 This methodology has been applied to introduce azide functionality in the enzyme Candida antarctica lipase B (CalB) that was subsequently coupled by means of a CuAAC reaction to poly(ethylene glycol) containing an alkyne terminus.22
Functionalizing molecular assemblies
The application of click chemistry in bioconjugation reactions not only works well on molecularly dissolved species, but can also be used to functionalize molecular assemblies. For example, amphiphilic block copolymers are capable of self-assembling into vesicular structures in solution. These polymer vesicles, also referred to as polymersomes, are spherical shell structures that exhibit remarkable stability and can be used to encapsulate a wide variety of compounds. For these nanocontainers to serve as drug delivery vehicles or nanoreactors, they would need to be equipped with targeting ligands or enzymes. To illustrate this approach, polystyrene-block-poly(acrylic acid) (PS-b- PAA) was prepared and the bromide terminus substituted for an azide moiety.23 Upon slow addition of water to a solution of the block copolymer in dioxane, this amphiphilic block copolymer self-assembled into polymersomes with the azide functionalities exposed on the outside of the vesicle. After extensive dialysis against water to remove the organic solvent, several alkyne-functionalized substrates, including enhanced green fluorescent protein (eGFP), were coupled to the exterior of the vesicles (Figure 4). The fluorescent behavior of the eGFP-tethered polymersomes was visualized by confocal laser-scanning microscopy, as illustrated in Figure 4. In a control experiment in which the addition of a copper-catalyst was omitted, no fluorescence was observed leading to the conclusion that eGFP was covalently bound to the vesicles.
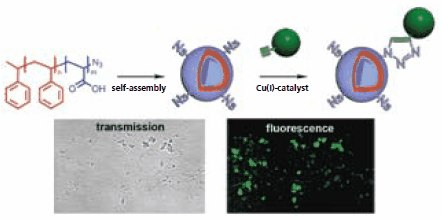
Figure 4. Schematic representation of the polymersome formation from azide functionalized polystyrene-block-poly(acrylic acid) and the subsequent functionalization of its periphery with enhanced green fluorescent protein using “click” chemistry, as visualized by confocal laser-scanning microscopy.23
In subsequent research, semiporous polymersomes composed of polystyrene-block-poly[L-isocyanoalanine(2- thiophen-3-yl-ethyl)amide] (PS-b-PIAT) were functionalized at the periphery with the enzyme CalB by using click chemistry.24 Since in this case the polar PIAT block could not be equipped with a desired alkyne moiety, an alkyne terminated polystyrene-block-poly(ethylene glycol) block copolymer was coaggregated within the vesicles, thereby providing a handle for further functionalization. The enzyme retained its activity after attachment to the polymersome.
Opportunities in biomaterial science
Biohybrid polymers have been recognized for many years as versatile materials for application in drug delivery, nanotechnology and bioengineering. Because pure biomolecules are susceptible to conformational changes which, in general, involve loss of function, bioconjugate research will be an important theme in the coming years, leading to new and improved material applications. In this respect, click chemistry has a promising future as it has been proven to be an efficient, specific and biologically compatible method for coupling two compounds. Together with parallel advances in polymer chemistry and protein engineering scientists now have a comprehensive toolbox with which to prepare biohybrid macromolecules with welldefined architecture and properties.
References
To continue reading please sign in or create an account.
Don't Have An Account?