Dendrons and Hyperbranched Polymers: Multifunctional Tools for Materials Chemistry
Introduction
Dendrimers are polymeric macromolecules composed of multiple perfectly-branched monomers radially emanating from a central core (Figure 1). The number of branch points increases upon moving from the dendrimer core to its surface and defines dendrimer generation. The branched topology confers dendrimers with several unique properties for materials applications:
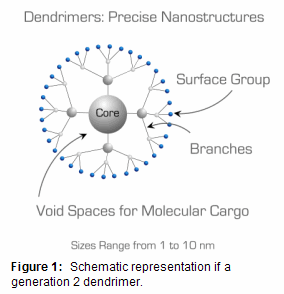
- Compact nm-scale dendrimer structure results in high solubility and low solution viscosity. This property lends dendrimers to applications as rheology (viscosity) modifiers.1
- Dense presentation of multiple terminal groups on dendrimer surface (multivalency), with number of surface groups increasing with dendrimer generation. Multivalency can be exploited as a means to achieve concentrated payloads of drugs or imaging labels chemically grafted to the dendrimer surface.2
- Core-shell molecular architecture with chemically distinct interior and surface. The core-shell architecture can be used to encapsulate and release molecules chemically incompatible with the environment external to the dendrimer: for example catalysts, drugs, or chromophores.3
We offer a wide range of monodisperse dendrimer molecules with four different chemical structures (PAMAM, phosphorous, polypropylenimine, polylysine) and numerous distinct terminal surface groups. To expand the range of dendritic tools for your materials chemistry research, we are pleased to offer two types of dendritic macromolecules: polyester dendrons and hyperbranched polymers based on 2,2-bis(hydroxymethyl)propanoic acid (bis-MPA) monomer.
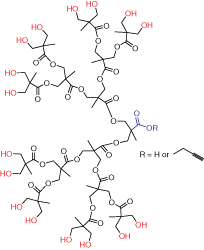
Figure 2.Generation 4 bis-MPA dendron
Dendrons
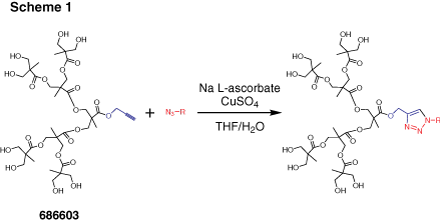
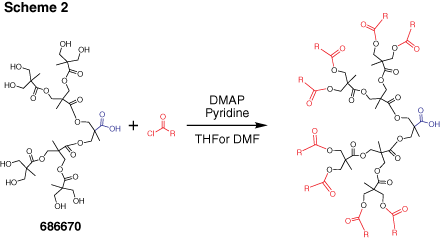
Dendrons (Figure 2) are monodisperse wedge-shaped dendrimer sections with multiple terminal groups and a single reactive function at the focal point. In addition to unique properties, described above, this gives you an option of orthogonal reactions utilizing the distinct focal point and surface groups. For example, it is possible to graft dendrons to a surface, to another dendron,4 or to another macromolecule.5 Our bis-MPA dendron products are available in three generations with alkyne or carboxylic acid focal point functions. Alkyne functions are suited for “click chemistry” catalytic cycloaddition to azides (Scheme 1) (see our list of available azides here). Carboxylic acids can be selectively reacted with amines, for example using EDC-mediated coupling. Multiple surface hydroxyl functions can be further derivatized, for example, by reactions with anhydrides or acyl halides (Scheme 2). Please refer to our Technical Bulletins listed in Product Table for detailed product information.
Hyperbranched Polymers
Hyperbranched polymers (Figure 3) are polydisperse dendritic macromolecules that possess dendrimer-like properties but are prepared in a single synthetic polymerization step. They are imperfectly branched and have an average (rather than precise) number of terminal functional groups, but are more cost effective than the perfect dendrimer products.
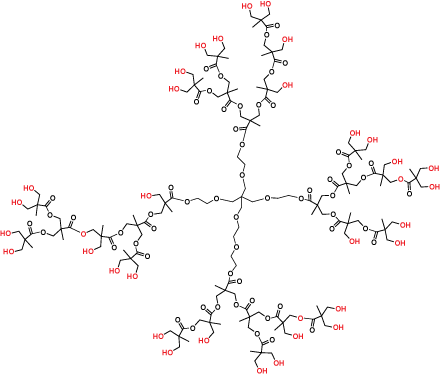
Figure 3.Hyperbranched bis-MPA polyester Generation 3 (Product No. 686581)
In applications tolerant of the imperfections, hyperbranched polymers will deliver the advantages of dendrimers at a much lower cost. Our bis-MPA hyperbranched polymer products are highly purified (> 97% product purity) and supplied as easy-to-dissolve lyophilized powders.
Features and Benefits
Unique features and benefits of the new hyperbranched polymer products include:
- Multiple hydroxyl (-OH) end-groups readily available for further chemical functionalization, for example by reaction with anhydrides and acyl halides
- Applicability as versatile precursors for hybrid dendritic-linear materials 6
- High degree of branching that results in high solubility and low viscosity (in solution and melt)
- Utility as polyfunctional initiators and cross-linkers for ring opening polymerizations and thermosets
- Transparency to light in the ultraviolet-visible wavelength region
All polyester dendrons and hyperbranched polymers are soluble in water and polar organic solvents (methanol, THF, DMF, DMSO). It has been demonstrated that bis-MPA dendritic macromolecules are biocompatible and biodegradable, with non-toxic degradation products. This makes them particularly well-suited for drug delivery and other biomaterials applications. 7
Product Table. Polyester Dendron and Hyperbranched Polymer Products
References
To continue reading please sign in or create an account.
Don't Have An Account?