Functionalized PEI and Its Role in Gene Therapy
Introduction
Gene therapy has become one of the most discussed techniques in biomedical research in recent years. Gene therapy is the treatment of a variety of diseases and genetic disorders by delivering genetic materials into cells. However, success of this treatment method is still limited due to the lack of safe and efficient carrier systems.
Poly(ethyleneimine) (PEI) has been extensively explored as a state-of-art gene carrier for in vitro and in vivo applications. PEI readily forms polyplexes with nucleic acids via electrostatic self-assembly. Polyplexes provide superior transfection efficiency due to the high buffering capacity of PEI, which is beneficial for endosomal escape of the gene payload.1 Despite these advantages, PEI is not degradable, lacks specificity, yields inherently instable complexes, and aggregates in the blood, resulting in high toxicity. There has been a significant focus on the modification of PEI and its corresponding effects on gene delivery. While low molecular weight PEI has been shown to be less cytotoxic in vivo than high molecular weight derivatives,2 low molecular weight PEI results in reduced transfection efficiency. Based upon these results, current research has focused on the design of degradable PEI derivatives using low molecular weight PEI and a variety of crosslinkers. Moreover, different polymers and ligands have been used to increase transfection efficiency and target specificity.
This review focuses on current advancements in the development of modified PEI derivatives as gene carriers for small interfering RNA (siRNA) and DNA and briefly describes different chemical modifications of PEI supported by in vivo results. Topics covered include PEI-coupled polymers to enhance polyplex stability, transfection efficiency, and the addition of targeting ligands and biodegradable linkages.
Modifications of PEI by Polymer Functionalization
Despite its unique properties, unmodified PEI suffers from cytotoxicity, lack of biodegradability, poor transfection efficiency of low molecular weight homologues, and insufficient target specificity. To obtain more efficient non-viral vectors for gene delivery, a substantial focus has been placed on PEI modifications in the last several years. Polymers, ligands, and chemical modifications have been used to improve the physicochemical properties of PEI, as well as enhance its biocompatibility and transfection efficiency. The modification of PEI with polymers to enhance transfection efficiency has attracted significant interest and utilizes a wide variety of polymers including oligosaccharides, poly(ethylene glycol) (PEG), poly(ε-caprolactone), and others. The most common and recent modifications are described briefly herin.
PEG
One of the first and most extensively investigated PEI modifications is the covalent coupling of poly(ethylene glycol) (PEG) (Figure 1). Since PEG is non-ionic and water-soluble, conjugation with PEG improves biocompatibility, reduces cytotoxicity, and increases circulation time in vivo.3 Mao et. al4 found that both the chain length and graft density of PEG strongly influence siRNA condensation and polyplex stability in vitro. While a tremendous number of studies have been performed in vitro, in vivo experiments showing the effects of PEG-PEI/siRNA complexes are essential for the validation of new materials. Merkel et al.5 investigated the delivery of PEGPEI polyplexes to the lungs of mice. Preliminary in vitro results suggested PEI/siRNA without PEG had the least immunogenicity and best stability, but these results were not supported by subsequent in vivo knockdown experiments. In the secondary experiments, PEG-PEI/siRNA complexes showed higher stability and elevated immune responses but no histological abnormalities, while unmodified PEI/siRNA complexes deposited PEI in the lungs and released the siRNA payload too early.
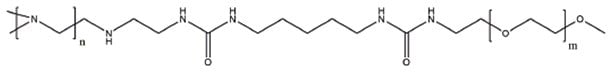
Figure 1.Example structure of a PEI modification with PEG.
Saccharides
Due to its biodegradability, biocompatibility, and low toxicity, chitosan is the most common polysaccharide used for grafting on PEI. To overcome its poor transfection efficiency, modified derivatives such as glycol chitosan have been attached to PEI polymers.6 This gene carrier reduces the cytotoxicity of PEI while enhancing endosomal escape and GFP transfection efficacy in HEK 293 cells. Apart from polysaccharides, oligosaccharides are frequently used to alter the pharmacokinetic properties of PEI. Gutsch et al.7 examined a set of different oligomaltose-PEIbased DNA and siRNA complexes for their biocompatibility and efficacy in vivo. They found oligomaltose-grafted PEI increases DNA delivery efficiency, decreases weight loss, abrogates hepatotoxicity, and abolishes lethality in mice in the stucdy when compared to free polymers. Another interesting approach is the use of cellulose nanocrystals (CNCs) as vehicles for siRNA delivery.8 These rodlike nanoparticles feature biodegradability, lack of cytotoxicity, and enhanced cellular uptake. In a recent report, CNCs were coated with a covalently-attached, cationic PEI shell capable of binding siRNA via electrostatic interactions. This new nanocarrier showed no cytotoxicity and good transfection efficiency compared to a commercial transfection reagent in vitro.
Polylactide
To increase hydrophobicity for better cell penetration and overall biodegradability, Abebe et al.9 designed triblock copolymers constituted of linear PEI, PEG, and poly(l-lactide) (PLLA). They prepared multi-layered micelles with PLLAPEI- PLLA as the hydrophobic core encapsulating the genetic material and PLLA-PEG-PLLA as the outer shell to ensure stability in aqueous suspensions. The resulting micelles show low cytotoxicity and high stability at neutral pH, but the outer shell was easily destabilized in acidic conditions, releasing the nucleic acids immediately.
Other Polymers
There are numerous other ways to modify PEI with different copolymers to yield better transfection efficiencies and reduced cytotoxicity. A multifunctional, cationic triblock copolymer based on PEG, poly(ε-caprolactone) (PCL), and PEI was designed for the delivery of siRNA.10 The hydrophobic PCL domain increased the affinity for the cell membrane, maintaining better internalization. Since this layer can also serve as a reservoir for hydrophobic substances, the system has the potential to be used for the simultaneous delivery of nucleic acids and hydrophobic drugs or dyes. These hydrophobic, amphiphilic nanocarriers showed superior transfection efficiency when delivered to the lungs of BALB/c mice via inhalation. PEI conjugates of single-walled carbon nanotubes can also be used to reduce PEI’s cytotoxicity while enhancing its transfection efficiency.11
This nanovector takes advantage of both the morphologybased, needle-effect cell penetration of carbon nanotubes and the endosomal escape ability of PEI. In addition to the enhanced transfection efficiency and low cytotoxicity, disulfide linkages were incorporated to empart biodegradability into the nanoconstruct. In comparison to 25 kDa PEI, the nanocarriers showed greater than 800-fold higher transfection levels in vitro.
Ligand-Modified PEI
An ideal gene delivery system should deliver intact nucleic acids without side effects, while also providing a basis for cell- or tissue-specific targeting. Many in vivo studies have demonstrated the inability of genetic material to enter the cell nucleus. To overcome this drawback and enhance targeting efficiency of PEI-based polyplexes, the polymer must be modified with distinct targeting ligands. Many different approaches have been reported; only select reactions will be described here.
Peptides
Specific peptide sequences that interact with receptors expressed predominantly by tumor cells can be included to facilitate cell and nucleus penetration. Hu et al.12 designed a PEI-based vector modified with the trifunctional peptide R18, which contains TAT (a cell penetrating peptide), RGDC (a cell adhesive peptide), and a nuclear localization sequence (NLS), to increase the nuclear import of genetic material. This nanovector showed controlled degradation, high buffer capabilities, and low cytotoxicity. Confocal fluorescence microscopy confirmed accumulation in the nucleus after cellular uptake. In addition, in vivo results in mice support the importance of the NLS fragment to increase the efficiency of nuclear transfection, compared to the complex without the NLS sequence or any peptide modification.
Neutralization of Surface Charge
The neutralization of PEI surface charge has been shown to increase biocompatibility and enhance cell internalization efficiency.13 Substitution of the primary amines of PEI with hydrazide groups was shown to allow for further modification with targeting ligands. Despite these modifications, the “proton sponge effect” remains unaffected due to the unmodified secondary and tertiary amines of poly(ethyleneimine). The resulting polymers were pH-sensitive and showed no cytotoxicity in human umbilical vein endothelial cells (HUVEC), suggesting that the primary amines found in PEI are primarily responsible for its cytotoxic effect. With the adhesive peptide RGD as the target ligand, these nanocomplexes were able to facilitate the uptake of siRNA into zebrafish hearts.
Folic Acid
Most tumor cells (and a subpopulation of macrophages) show an overexpression of folate receptors on their surface. Following fundamental work by Abebe et al.,9 Mohammadi et al.14 used folic acid-decorated micelles to target activated macrophages in inflamed joints (Figure 2). The inner core of the micelles consisted of PLLA-PEI-PLLA block copolymers, while the outer layer was prepared with different ratios of PLLA-PEG-PLLA and folic acid (FA)-PEG-PLLA. 75% FA-PEG-PLLA and 25% PLLA-PEG-PLLA were found to be the optimal formulation for effective plasmid DNA encapsulation, cellular uptake, and macrophage targeting. With this composition, a significantly higher GFP expression was achieved in activated RAW 264.7 macropahges ex vivo, compared to non-targeting micelles and non-activated macrophages.
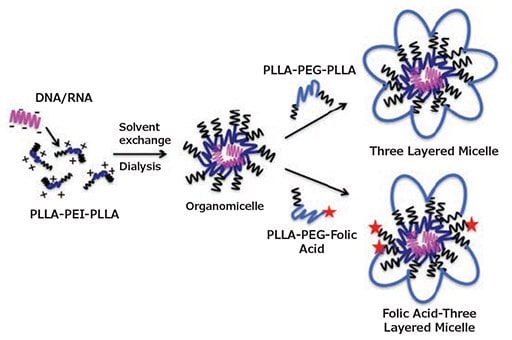
Figure 2.Formulation of non-targeted and targeted three-layered micelles in a 2-step process. Depending on the use of PLLA-PEG-PLLA only or blends of PLLA-PEG-PLLA with PLLA-PEG-FA for the outer layer, non-targeted or folate-receptor targeted three-layered micelles are obtained, respectively. Reprinted with permission from Mohammadi et al.14 Copyright 2016 Elsevier.
Transferrin
A more established and successful approach towards efficient polyplex shielding, utilizes the highly hydrophilic, negativelycharged serum glycoprotein transferrin (Tf). This ligand combines both an intrinsic stealth effect and targeting towards transferrin-receptor expressing cells,2 eliminatng the need for a PEG-shell to shield the positive charge of the Tf-PEI/ DNA complex. This gene delivery system not only exhibits decreased erythrocyte aggregation compared to its PEGylated derivative, but also reduced cytotoxicity (Figure 3). Moreover, the biodistribution pattern was shifted from liver and lung, as typical for unshielded complexes, to transgene expression predominantly in the targeted tumor in vivo. Thus, transferrin can be used as a shielding and targeting component, to efficiently deliver genetic material into tumor cells.
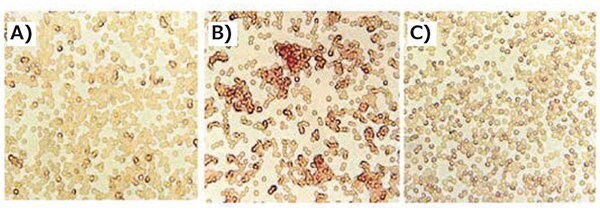
Figure 3.Incorporation of transferrin decreases erythrocyte aggregation. Fresh murine erythrocytes were incubated with PEI/DNA or Tf-PEI/PEI/DNA complexes (N/P = 4.8) for 1 h at 37 °C. A) Tf-PEI/PEI/ DNA, B) PEI/DNA, C) control. Adapted with permission from Kircheis et al.2 Copyright 2002 Nature Publishing Group.
Chemically Modified PEI
Poly(ethyleneimine) has been regarded as the gold standard of polymeric gene carriers due to its high buffering capacity for the endosomal escape of genes, but is severely limited by its high cellular toxicity. The cytotoxicity of PEI depends on its degradability, molecular weight, and structure. In this section, we describe two of the most common linkages incorporated into PEI derivatives to yield degradable analogs.
Ester Bonds
PEI derivatives containing ester bonds can be prepared by the Michael addition of PEI to diacrylates, which act as crosslinkers. Wang et al.15 coupled branched, low molecular weight PEI to different Pluronic® diacrylates. The poly(ester amine)s (PEA) obtained were examined thoroughly in vitro and in vivo. PEAs showed limited cytotoxicity toward C2C12 and CHO cells, even in high concentrations, whereas cell viability dropped to less than 15% when using standard PEI (25 kDa). Furthermore, a significant improvement in gene delivery efficacy of CHO, C2C12, and HSkM cells was achieved, as well as effective transfection in mdx mice.
Disulfide Bonds
The concentration of glutathione and other reducing agents is much higher in the cytoplasm than in the plasma, imparting a high intracellular reduction capacity compared to the extracellular milieu. Redox-sensitive materials typically incorporate degradable disulfide linkages in their backbone, as these bonds degrade in a reducing environment. Cellular uptake with fast intracellular siRNA release in vitro was observed when using a disulfide-crosslinked, highly-branched PEI in comparison to unmodified linear and branched PEI.16 Neu et al.17 used a low molecular weight crosslinking reagent, dithiobis(succinimidyl propionate) (DSP), to generate crosslinked PEI polyplexes without the additional steric stabilization of hydrophilic copolymers (Figure 4). These polyplexes were comparable in size and DNA condensation properties, and were efficiently taken up by cells in vitro with a redox-triggered DNA release. In vivo experiments in mice showed increased blood concentrations. A luciferase assay demonstrated enhanced liver expression and reduced unwanted 0 h 4 h 24 h 48 h 72 h 96 h 120 h lung transfection. These results show that DSP-crosslinked PEI creates reducible disulfide bonds that can prolong circulation time and maintain transfection efficiency. Additionally Zhang et al.18 enhanced DNA delivery and transfection efficiency while reducing cytotoxicity (in vitro and in vivo) by conjugating a reducible, disulfide-linked PEI to biocompatible Pluronic®.
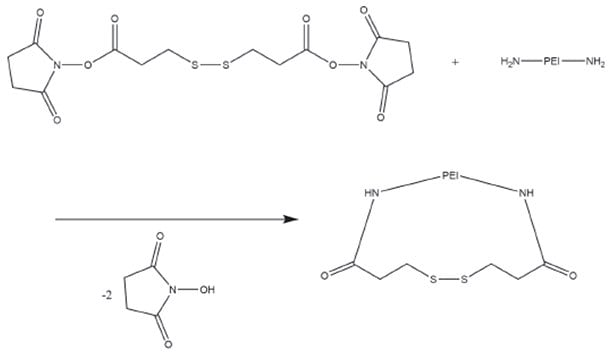
Figure 4.Reaction scheme for the conversion of PEI primary amines with dithiobis(succinimidyl propionate) (DSP). The resulting disulfide bonds are easily cleaved by reducing agents.
Conclusion
As one of the most studied non-viral vectors for nucleic acid delivery, PEI holds significant potential for gene therapy applications. Due to its ability for RNA/DNA condensation, cellular uptake, and endosomal escape, poly(ethyleneimine) is a suitable candidate for the in vivo delivery of therapeutic nucleic acids. Although non-modified PEI-based carriers have been considered the gold standard, PEI features several key drawbacks such as cytotoxicity, lack of target specificity, and transfection efficiency. Considerable efforts have been made to assess different modifications of PEI and overcome these drawbacks. Promising results regarding target specificity, transfection efficiency, and biodegradability have been achieved, but there is still significant room for improvement. In the past two decades, substantial advances have been made using degradable PEI-based delivery systems, improving both safety and potency. Scientists have also developed multifunctional polyplexes for receptor-mediated gene delivery in vivo (Figure 5),19 some with the capacity to be used in diagnosis (bioimaging) and therapy, via gene delivery at the same time (theranostics).20 However, further in vivo studies are needed to explore additional pharmacodynamics, pharmacokinetic, and immunogenic properties; improving the potential of PEI-based gene carriers for use in clinical applications.
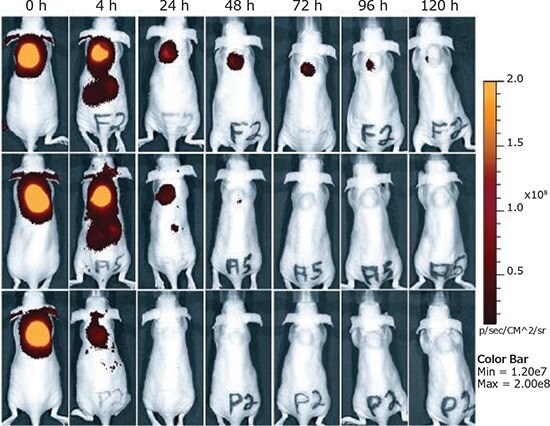
Figure 5.Intratumoral injection of siRNA polyplexes. Retention of targeted polyplexes at the tumor site determined by NIR fluorescence bioimaging. Folic acid-targeted polyplexes (top), untargeted polyplexes (center), and equal amounts (25 μg) of free Cy7-labeled siRNA (bottom). Adapted with permission of Dohmen et al.19 Copyright 2012 American Chemical Society
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?