Nonporous Silica Nanoparticles Applications
Section Overview
- Introduction
- Silica-Polymer Nanocomposites
- Nanomedicine
- Imaging
- Removal of Dyes from Wastewater
- Enhanced Oral Delivery of Macromolecules
- Nano Chromatography / Ultra-Efficient Chromatography
- Lithium Ion Batteries — Gel Electrolytes Based on Lithium-Modified Silica Nanoparticles
- LCD Spacers
- References
Introduction
Spherical silica is one of the most important and widely used materials globally, whether as a simple additive in everyday products, such as toothpaste and cement, or in more advanced applications, such as drug delivery, chromatography, and sensor technology. Traditionally, nonporous amorphous silica nanoparticles (SiNPs) are mainly employed as additives in cosmetics, printer toners, packaging, and imaging. However, with the development of more monodispersed and size-controlled particles, nonporous SiNPs are now actively being investigated as promising nanocarrier systems for delivering drugs to various human tissues, and as novel materials for smart plastics. Today, SiNPs are commercially available in a range of sizes, from 100 to 700 nm (Figure 1). Owing to their large surface area-to-volume ratio, stable chemical structure, and ease of surface modification, SiNPs have become increasingly attractive as promising candidates for exploring a whole spectrum of new research areas, some of which are reviewed in this article.
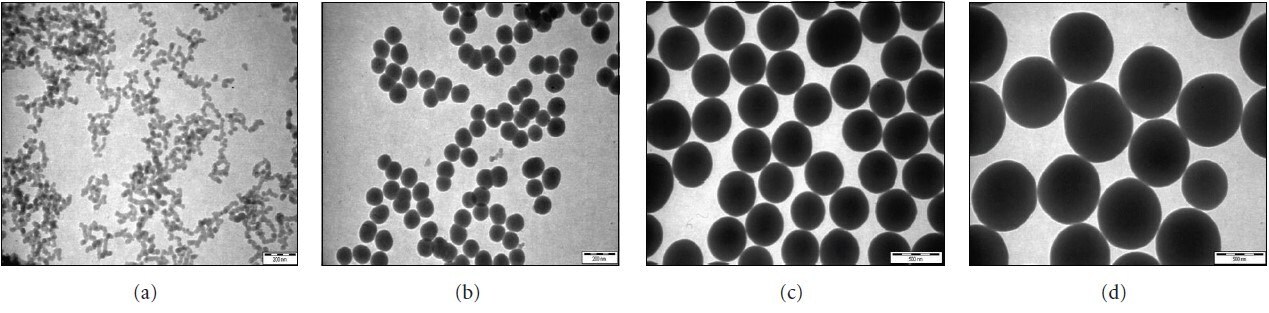
Figure 1.Typical TEM of the size range (100–700 nm) of SiNPs commercially available.
Silica-Polymer Nanocomposites
The application of silica nanoparticles as fillers in the preparation of nanocomposite polymers has drawn much attention due to the increased demand for new materials with improved thermal, mechanical, physical, and chemical properties. Recent advances in the synthesis of monodispersed nanoparticles with narrow-size distribution by the sol-gel method have significantly boosted the development of silica-polymer nanocomposites. One of the prominent applications of silica nanoparticles is as fillers or reinforcement in advanced composite materials. A crucial aspect of silica-polymer nanocomposites is the ability to attain homogenous filler dispersion, which determines the overall performance of the nanocomposites. Chemical modifications of the silica (filler) surface and an effective mixing method are two main routes leading to homogenous filler distribution.
On the other hand, the performance of a nanocomposite also depends on the type of polymeric matrix used. Due to the large boundary surface created by the silica nanoparticles (fillers), it is possible to produce silica-polymer nanocomposites with new and improved properties. The advantages of nanoparticles include efficient reinforcement with excellent mechanical strength, heat stability, reduced shrinkage, thermal expansion and residual stress, improved abrasion resistance, and enhanced optical and electric properties. The decrease of particle size below 100 nm enables good optical transparency, especially for silica. In addition, nanoparticles offer various property enhancements at lower loadings due to a higher surface-to-volume ratio than the conventional particles. Therefore, nanocomposites offer exciting properties that allow their use in automotive, aerospace, electronic, and engineering applications. Table 1 summarizes various types of silica-based nanocomposites together with the resulting properties as reported in the literature.
Nanomedicine
Nanomedicine, the use of nanotechnology for medical applications, has undergone rapid development in the last several decades. Nanomedicine aims to design and synthesize drug delivery vehicles that can carry sufficient drug loads, efficiently cross physiological barriers to reach target sites, and safely and sustainably cure diseases. Numerous organic nanomedicines, including liposomes, drug-polymer conjugates, dendrimers, polymeric micelles, and nanoparticles (NPs), have been extensively studied as drug delivery systems. Silica nanoparticles have attracted significant interest because of their unique properties amenable for in vivo applications, such as their hydrophilic surface favoring protected circulation, versatile silane coupling chemistry, excellent biocompatibility, and ease of large-scale synthesis. Table 2 and Figure 2 illustrate the broad range of nanomedicine-specific applications of SiNPs.
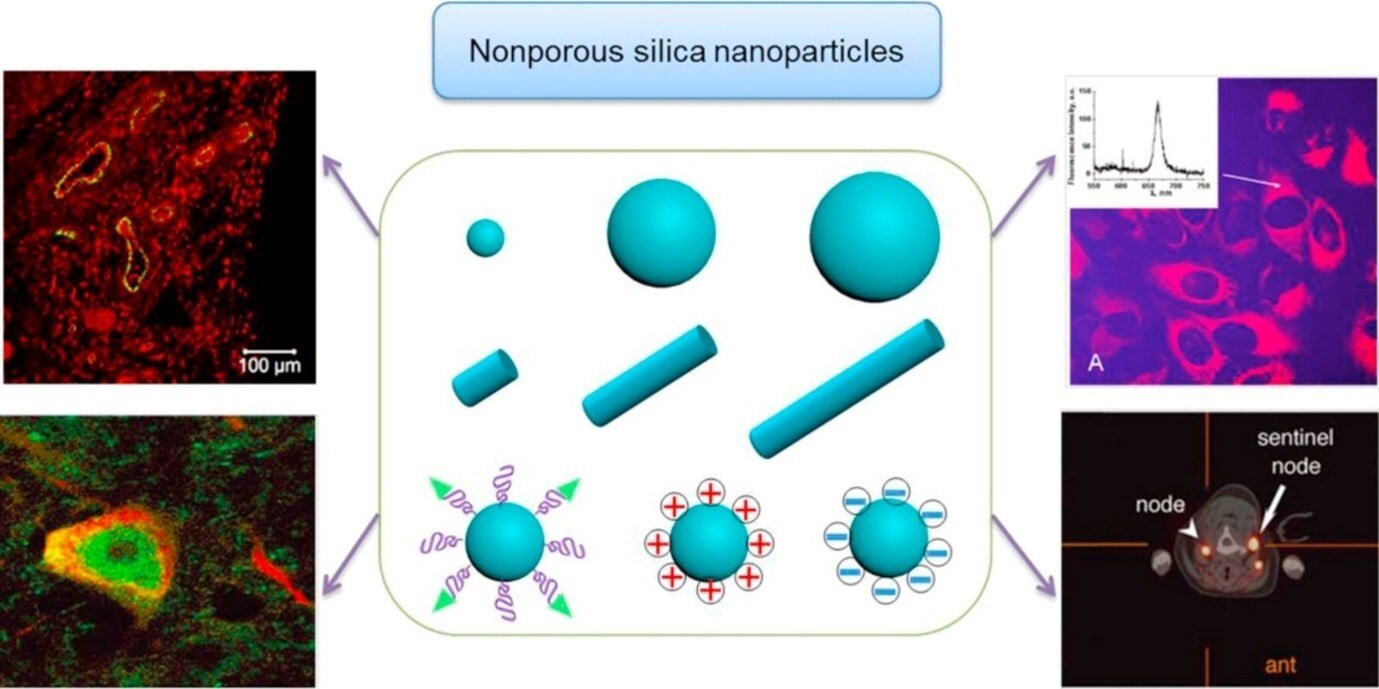
Figure 2.SiNPs used in nanomedicine. (Reprinted with permission from Reference 1, Frontiers Media S.A., 2018.)
Imaging
Nanotechnology offers unprecedented opportunities for addressing current challenges in cancer diagnosis. SiNPs carrying diagnostic probes can provide structural and metabolic information from disease sites. NP-based imaging techniques can markedly improve the detection and staging of cancers and their metastases. Besides the application for delivery of therapeutic agents, SiNPs are also actively employed as a platform for incorporating contrast reagents for molecular imaging. Their versatility and robust chemistry allow easy adaptation of diagnostic probe-doped silica NPs for molecular imaging techniques, including optical imaging (fluorescence and bioluminescence), MRI, radionuclide imaging (positron emission tomography (PET) and single-photon emission computed tomography (SPECT)), computed tomography (CT), ultra-sound, photoacoustic imaging, and Raman imaging. Single and multiple modal imaging techniques based on silica NPs have been actively explored during the past two decades, and many types of multi-functional silica NPs with applications for cancer diagnosis have been developed (Figure 3).
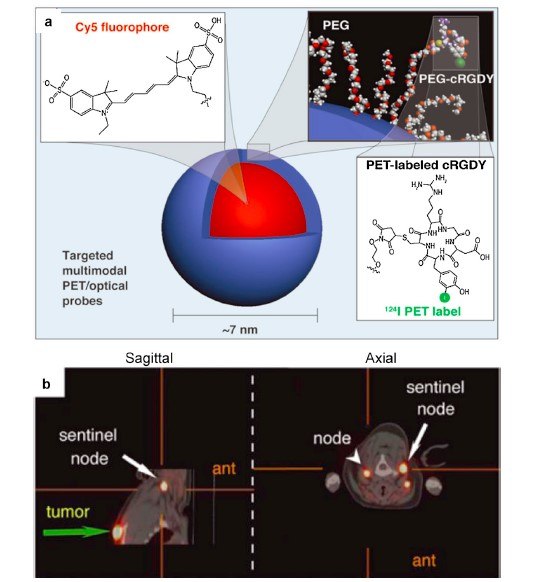
Figure 3.Multimodal silica nanoparticles for targeted cancer diagnosis in a model of human melanoma. (Reprinted with permission from Reference 2, Royal Society of Chemistry, 2009.)
Removal of Dyes from Wastewater
Traditional water treatment and purification methods, such as mechanical separation, filtration, flocculation, coagulation, and chemical treatment or disinfection, have been practiced for many decades. Although some of these techniques still retain their importance, there is a need to invent and test new water treatment technologies that are faster and more efficient than conventional methods. Recently, nanotechnology for water remediation has received increased attention due to the invention of new functional nanomaterials with high efficiencies to entrap various pollutants from water. For example, silica and silica-based hybrid nanoparticles are currently investigated with great interest for the removal of dyes from water (Figure 4). The presence of surface silanols which can complex the dyes, ease of grafting of additional functionalities to improve the complexation, the possibility of photocatalyst grafting for dye degradation, and good chemical stability are the reasons for the excellent efficiency of silica nanoparticles to remove dyes from water.
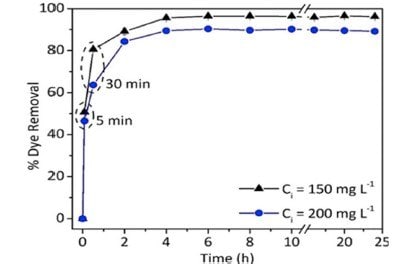
Figure 4.Effect of time on the removal of acid orange 8 by silica nanoparticle (200 nm) adsorbent. Conditions: 25 °C; initial concentration: 150 and 200 mg/L; adsorbent mass: 1.0 g/L. (Reprinted with permission from Reference 3, American Chemical Society, 2009.)
Enhanced Oral Delivery of Macromolecules
Oral drug delivery is one of the most preferred routes of therapeutics administration due to high patient compliance, non-necessity for trained personnel, and lower costs. Despite the benefits, the oral delivery of many small molecules and most macromolecules remains very challenging. Macromolecular drugs are either originally produced from living organisms or engineered synthetically, and the majority of FDA-approved macromolecules are peptides, antibodies, and antibody fragments. The recent improvements in genomics, proteomics, and biotechnology in peptide synthesis have led to a huge surge in the production of monoclonal antibodies, enzymes, and cytokines. They offer a set of specific pharmacological functions, relatively low incidence of cross-reactivity, and immunogenicity. Thus, there is a compelling need for using advanced formulation strategies to administer these challenging therapeutics via the oral route. Numerous strategies, including pH-sensitive capsules, oral microneedle-based delivery, and intestinal patches, have been explored to tackle this challenge. Most of these strategies overcome gastric denaturation but remain inadequate in surpassing the physiochemical hurdles, limiting epithelial permeation and bioavailability. In recent years, SiNP-based delivery systems have been shown to overcome both the gastric barrier and physicochemical challenges in the gut, due to their high surface-to-volume ratio, controlled release, cellular transport mechanisms, and enhanced oral bioavailability of payloads. SiNPs are particularly interesting because of their highly tunable surface chemistry, proven biocompatibility, cost-effective synthesis, and chemical and enzymatic stability.
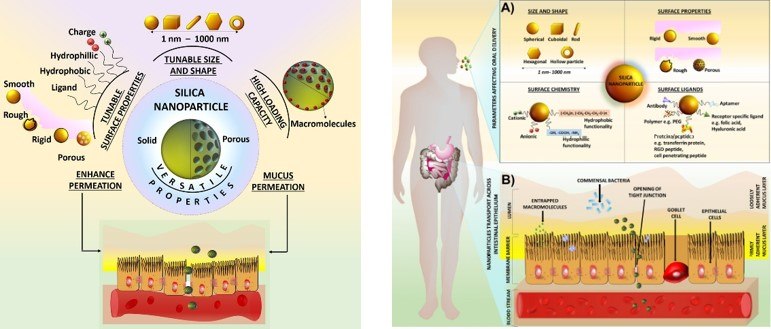
Figure 5.Schematic diagram of (A) Parameters affecting oral delivery of silica nanoparticles across GIT, and (B) Potential mechanisms of transport of silica nanoparticles across the intestinal epithelium. (Reprinted with permission from Reference 4, MPDI, 2019.)
Nano Chromatography / Ultra-Efficient Chromatography
SiNPs (400 nm) packed into narrow-bore HPLC columns offer a new and exciting prospect in chemical separation and purification. Recent work has shown a phenomenon known as “slip flow” occurs in colloidal crystals made of 470 nm silica spheres that are chemically modified with hydrocarbon, giving enhanced volume flow rates, and a narrower distribution of fluid velocities. This phenomenon has lead researchers to separate a monoclonal antibody from its aggregates in only 40 seconds, representing a 10-fold increase in speed. Slip flow, thus, has profound implications for protein chromatography. Figure 6 shows a photograph of a cross-section of a cleaved capillary packed with 470 nm particles. The opalescence indicates crystalline domains. The SEM image confirms face-centered cubic (fcc) domains, which is expected for colloidal crystals, and the domain sizes are consistent with the photograph.
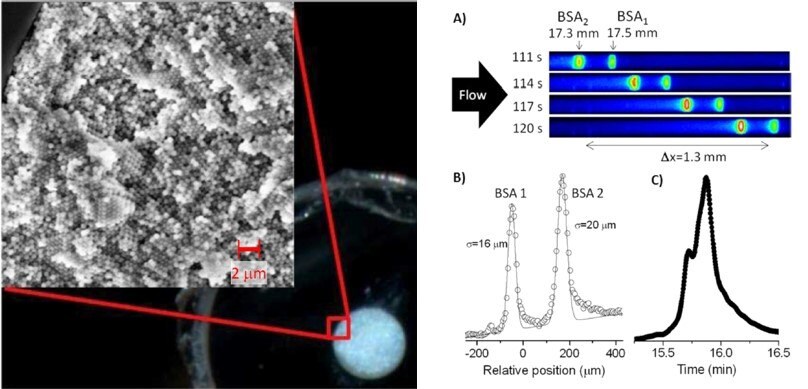
Figure 6.Photograph of the cross-section of a packed 75 μm i.d. capillary. The SEM image shows the individual 470 nm particles. Separation of labeled BSA. (A) Time series of fluorescence micrographs over the field of view. (B) Plot of the image data from the last frame of panels A: data points (O) and Gaussian fit. (C) Gradient elution chromatogram of the same protein sample using a 50-mm long commercial UHPLC. (Reprinted with permission from Reference 5, American Chemical Society, 2012.)
Lithium Ion Batteries — Gel Electrolytes Based on Lithium-Modified Silica Nanoparticles
Lithium-modified silica nanoparticles (Li-SiNPs) were synthesized and used as a single-ion lithium conductor source in gel electrolytes. Research showed that the Li-SiNPs exhibited good compatibility with DMSO, DMA/EC (a mixture of N,N-dimethyl acetamide and ethylene carbonate), and the ionic liquid N-methyl-N-propyl pyrrolidinium bis(trifluoromethylsulfonyl)amide [C3mpyr][NTf2], producing several gel electrolytes based on Li-SiNPs. Investigation of these gel electrolytes using DSC, solid-state NMR, conductivity measurements, and cyclic voltammetry demonstrated that they exhibited conductivities as high as 10−3 S/cm at room temperature. Electrochemical tests showed that some of these materials were promising for use as lithium-conductive electrolytes in electrochemical devices, with high lithium cycling efficiency evident, as shown in Figure 7.
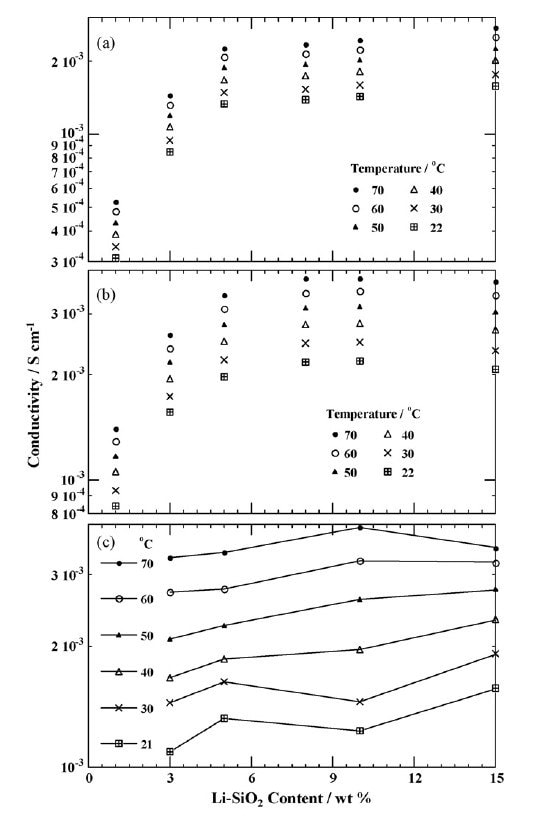
Figure 7.Conductivity as a function of Li-SiO2 content for the gel samples of (A) Li-SiO2 (1.1 mol Li/kg) in DMA/EC, (B) Li-SiO2 (3.7 mol Li/kg) in DMA/EC and (C) Li-SiO2 (1.1 mol Li/kg) in DMSO. (Reprinted with permission from Reference 6, Elsevier, 2007.)
LCD Spacers
The development of silica particles of nanometer to micrometer sizes has also attracted much attention in electronics. One of the applications of these silica particles is their use as a liquid crystal display (LCD) spacer. To use silica particles as spacers, they must be monodisperse, have high purity, and be easy to functionalize. The LCD panel is constructed by two glass substrates with deposited indium tin oxide (ITO) electrode film. The space between the two glass substrates (the cell gap) is filled with liquid crystals. Spacer particles maintain the cell gap between the two glass substrates, as shown in Figure 8. The highly uniform thickness of the liquid crystal layer is crucial for maximizing the display characteristics of the LCD. Currently, divinylbenzene (DVB) resin is commonly used as the spacer; however, DVB is easily compressible compared to silica, as shown below. Consequently, further development and refinement of SiNPs can be the key to great breakthroughs in electronics.
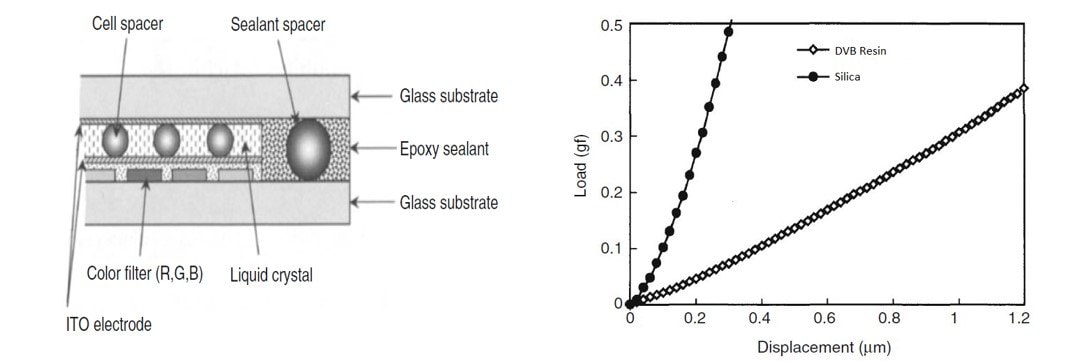
Figure 8.(A) Schematic illustration of the cross-section of an LCD cell. (B) Load–displacement curves of the silica particles compared with the DVB resin spacer. The loading speed is 0.27 gf/s. (Reprinted with permission from Reference 7, Springer, 2018.)
References
To continue reading please sign in or create an account.
Don't Have An Account?