Materials Design Concepts for Efficient Blue OLEDs: A Joint Theoretical and Experimental Study
Introduction
Since their discovery,1 organic light emitting devices (OLEDs) have evolved from a scientific curiosity into a technology with applications in flat panel displays and the potential to revolutionize the lighting market. During their relatively short history, the technology has rapidly advanced, and device efficiencies have increased more than 20-fold, approaching the theoretical limit for internal quantum efficiencies.2-4 At this point, OLED research moves towards optimization of manufacturing processes, drive circuitry, light extraction, and overall cost reduction. However, discovery of organic materials that provide both operational stability and high efficiency for the devices still remains one of the biggest challenges, particularly for blue emission. In this article, we will describe our approach to design functional OLED materials to meet the complex criteria set forth by device performance goals.
Design of Ambipolar Host Materials for Blue Phosphorescent OLEDs: From Theory to Experiment
The electronic criteria in a given OLED material include charge transport type, charge mobility, boundary orbital energies, and the triplet exciton energy. Figure 1 shows a schematic of a typical OLED energy diagram that sets the requirements for the position of the boundary orbital energy levels in each type of layer forming the device.
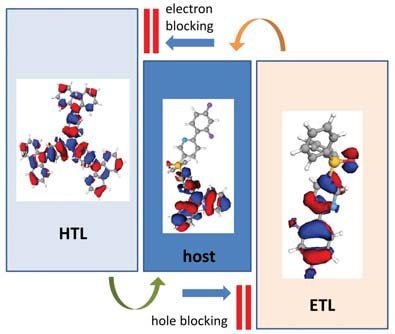
Figure 1.Diagram illustrating the OLED materials design criteria.
The energy diagram demonstrates that hole transport layer materials (HTL) need to have the HOMO level aligned with the corresponding HOMO level of the host to assure the hole flow into the emissive zone with minimal barrier for injection, whereas the HTL LUMO has to be sufficiently high to prevent electron leakage from the host into the HTL. A similar set of rules, but with the opposite sign, exists for the interface of the host with the electron transport layer (ETL): The LUMO levels need to be aligned, and the ETL HOMO sufficiently deep to provide charge confinement. Triplet exciton energies of the materials in both charge transport layers should be significantly higher than the highest triplet level of all the emitters to prevent emissive exciton quenching. The triplet energy constraints also apply to the host materials, but with the requirements less stringent compared to those of hole and electron transport molecules.5 In addition, the positions of the HOMO of the HTL and LUMO of the ETL will have to match the work functions of both electrodes to minimize charge injection barriers.
The set of energy alignment requirements in a given material can be satisfied by building it from molecular building blocks that carry the desired electronic properties. More often than not, synthesis and devicegrade purification of an OLED material are quite resource-intensive. The initial search for an appropriate structure can be initiated based on the known properties of molecular building blocks and chemical intuition. However, methods for screening the proposed materials before synthetic attempts are very valuable. Once we have selected a class of molecules that shows promise for use in OLEDs, the screening and down-selection process involves computational analysis to determine electronic properties of the molecules. Even though the absolute energy values that the computational methods of quantum chemistry can typically provide for relatively large molecules are different from those measured spectroscopically, the information extracted from these calculations is invaluable. For example, trends in electronic properties for large arrays of materials can be used to downselect to a narrower range of suitable candidates to synthezise. In Figure 2, we overview a class of host materials obtained as a result of such a downselection approach. The materials are constructed from two types of building blocks, the combination of which provides the host with the ability to transport both electrons and holes. The electron transporting groups are aromatic phosphine oxides and pyridines, whereas either carbazole or arylamine is responsible for hole transport. Succeded by computational evaluation, these materials were then synthesized, characterized, and used in actual devices.6,7 Table 1 summarizes the computed and experimental electronic properties of the host materials shown in Figure 2

Figure 2.Functionalized phosphine oxide based host materials.
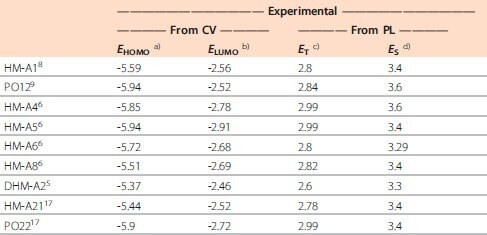

Table 1.Computed and experimentally determined electronic properties of the host materials depicted in Figure 2. (All values are in eV.)
a) Calculated from the oxidation potential using the equation EHOMO = –1.4*Eox – 4.6 eV.10 b) Calculated from reduction potentials using a bicarbazole biphenyl (CBP) LUMO as a reference.11 c) Estimated from the photoluminescence of frozen dichloromethane solutions at 77 K. d) Estimated from the intersection of the room temperature solution absorption and fluorescence spectra. e) Performed using NWChem computational package12 at the B3YLP/6-31G* level. f) Estimated from literature.29 g) The lowest energy transition T1 ← S0. h) The lowest energy non-zero transition S1 ← S0.
Comparison of theoretically and experimentally obtained values of molecular energy levels indicates the usefulness of the computations in predicting trends in how individual properties change. The theoretical Density Functional Theory (DFT) method slightly overestimates the orbital energies. However, the absolute values of the experimentallyobtained energies, measured via cyclic voltammetry (CV) in solution, have significant inaccuracy since the test method includes interactions with the solvent and electrodes, not present in operating OLEDs. Therefore, even though stand-alone numbers produced by either of the methods are of limited use, the relative positions of the energy levels in a series of molecules are useful in evaluating the suitability of a given compound as an OLED material compared to its derivatives. The experiments that were based on theoretical predictions resulted in the synthesis of a series of host materials that allowed for matching of the host properties with other layers in a specific device configuration. These host materials were then evaluated in OLEDs. We have demonstrated previously that the phosphine oxide moiety can be used as the building block for electron transport materials.13-16 The phosphine oxide-based compound 2,8-bis(diphenylphosphoryl)dibenzothiophene (PO15) is our standard electron transport material due to its favorable electronic structure and relatively high electron mobility. Its LUMO energy level determined by CV is -2.85 eV, which puts PO15 in good energy alignment with most of the host materials shown in Figure 3. Its deep HOMO level also provides adequate hole blocking in all cases.
On the other hand, the energy match between 1,1-bis[(di-4-tolylamino) phenyl]cyclohexane (TAPC) (EHOMO = -5.1 eV, ELUMO = -1.7 eV)5 and some of the hosts such as PO22 is less favorable, which results in decreased charge balance in the emission zone and increased barriers for the hole transport through the interface. This contributes to the EQE decrease in devices with certain hosts such as PO22 (Figure 3).

Figure 3.External quantum efficiency vs. current density plots for the devices with host materials from Table 1 and Figure 2, and the corresponding device structure.
Charge Transport in an Organic Material and Reorganization Energy
Even when energy alignment requirements are met, the charge transport within the host remains the main factor that influences charge balance. It has been shown that the charge balance in the emission zone affects the quantum efficiency of the phosphorescent device.18 To maintain such charge balance, a host material has to support transport of charge carriers of both signs. In addition to the energy level alignment, it is the transport in the host and the charge balance in the emission zone that result in devices with different efficiencies as shown in Figure 3.
In addition to thermodynamic effects that define the energy level alignment, the tools of computational quantum chemistry can help predict kinetic parameters that govern charge transport in OLED materials. Here, we attempt to build a library of molecules with high mobility using computed reorganization energies.
Charge transport in organic p-conjugated materials at room temperature occurs via a hopping-type mechanism.19-21 For OLEDs, this mechanism is described as a self-exchange transfer process. The holetransfer process is described as M + M+ → M+ + M, where M represents a neutral species undergoing charge transfer and M+ the species containing the hole. Using the standard Marcus model,22-24 we assume the mobility of a hole or an electron is dominated by the internal reorganization energy, (λ).25,26 The internal reorganization energy for hole and electron transfer can be expressed as follows:
λ(hole/electron) = λ1 + λ2 = (E(1) 1 - E(0) 1) + (E(0) 2 - E(1) 2)
As shown in Figure 4, E(0) 1 and E(1) 2 are the energies of the neutral and charged (cation/anion) species in their lowest energy geometry, whereas E(1) 1 and E(0) 2 represent the energies of the neutral and charged (cation/anion), which corresponds to the geometries of the charged (cation/anion) and neutral species, respectively.

Figure 4.Energy vs. Reaction (Q) coordinate scheme to illustrate reorganization energy calculations. See text for notations.
Smaller reorganization energies allow for lower energy barriers in the elementary step of electron transfer reactions. Such a reduced energy barrier results in higher charge mobility and is consistent with published reports.27 The correlation between our computed reorganization energies and experimentally determined hole mobilities available in published literature28 for a series of commonly used hole transporting materials for OLEDs is shown below in Figure 5. Though the trend is not perfectly linear, it is evident that as the reorganization energy decreases, the charge mobility increases. As expected there is good agreement between computed and experimentally determined values.

Figure 5.Time of flight mobilities(27) vs. reorganization energies computed in this work for common OLED hole transport materials.
Case Study: Host and Electron Transport Organic Materials with Deep LUMO Levels
Next, we will illustrate the use of the tools developed to design a family of host and electron transport/hole blocking materials with predetermined electronic properties. Sometimes it is desirable to have a deep LUMO in a host material to match a specific ETL and/or reduce the possibility of electron leakage into the HTL. On the other hand, the reasons for an ETL material to have a deep LUMO level can include energy level alignment with a specific cathode work function or ability to utilize n-doping by compounds with reasonable chemical stability. The approach to building host and ETL materials with deep LUMO levels can be based on the general principles described above for the design of ambipolar hosts. In this case study, the functional groups comprising the target molecule should provide a) the needed charge transport properties (ambipolar for the hosts, and electron-transporting for the ETLs), and b) electronic effects that allow for energy level shift. Figure 6 lists the prospective candidates that may satisfy the charge transport and LUMO alignment conditions.
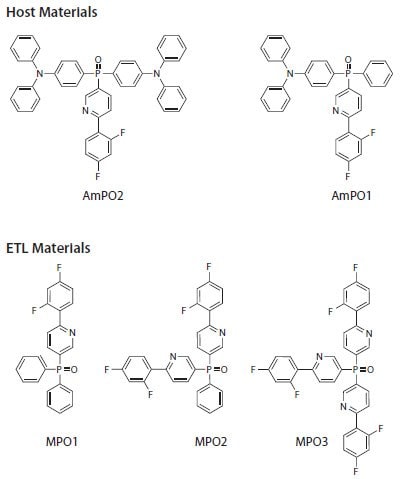
Figure 6.Difluorophenylpyridine-substituted phosphine oxide (DFppy-PO) host (top) and ETL (bottom) materials.
The chemical structures in Figure 6 were subject to geometry optimization, time-dependent DFT analysis to estimate the triplet level positions,29 and reorganization energy calculations,30 which allowed for prediction of the device-relevant molecular properties. We chose to build the molecules from the electron-transporting phosphine oxide moieties, hole-transporting arylamines, and difluorophenylpyridine (DFppy) groups that will provide the desired LUMO shift. The boundary molecular orbitals included in Figure 7 demonstrate the effect of this building strategy.

Figure 7.Computed electron density maps and orbital energies for the DFppy-PO host and ETL materials.
The two materials on the left (AmPO1 and AmPO2) are the hosts that should provide ambipolar charge transport. Their HOMO is localized on the arylamine fragment, whereas the LUMO is entirely defined by the dFppy unit. Therefore, the goal of providing a deep LUMO level for these materials is achieved by the choice of a functional group with a deep LUMO on which the LUMO of the entire molecule will be localized. The same holds true for the LUMO levels of the three ETL materials shown on the right in Figure 7. Unlike the hosts, there are no functional groups with shallow HOMO levels on the ETL molecules. In fact, both boundary orbitals of the ETLs are localized on the difluorophenylpyridine fragment. The HOMO energy of difluorophenylpyridine is quite low. It translates into good hole-blocking properties expected from the dFppy-based ETLs discussed here. The theoretically predicted electronic properties for the dFppy-substituted hosts and ETLs are listed in Table 2 below.
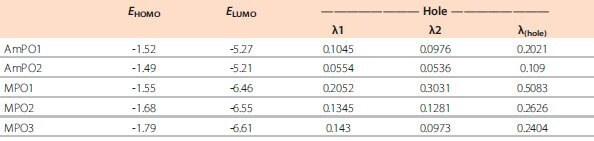
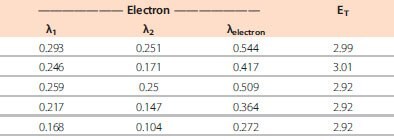
Table 2.Computed energy levels and reorganization energies for dfppy-PO host and ETL materials. (All values reported in eV.)
Conclusions
We reported on the methodology to design functional organic materials for OLEDs. Host and charge transport materials can be synthesized by combination of molecular building blocks that provide the necessary electronic properties. We showed how the correlation between theoretical predictions and the measured electrochemical and photophysical data allow for streamlining the OLED material design and down-selection process with examples from our recent research.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?