Early Microbial Detection with Milliflex® Rapid System 2.0
Sterility testing is a mandatory microbiology test required by cGMP for pharmaceutical products, medical devices, and other materials, to ensure the products are completely sterile and safe to use. Sterility tests are crucial for quality control and assurance of sterility of a product. Rapid sterility is an alternative test method to the USP Chapter <71>, Ph.Eur. 2.6.1, and JP 4.06 sterility tests that allow for shorter incubation times and faster results as compared to the traditional sterility testing method. The reduction in test time ensures faster release of sterile drug products and quicker corrective actions setting in case of failure.
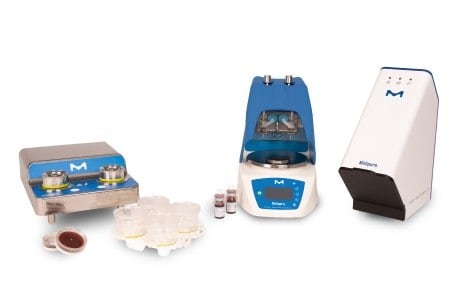
Milliflex® Rapid System 2.0 is an automated system for rapid and accurate microbial detection. Suitable for rapid sterility, bioburden, in-process, and product release testing, the Milliflex® Rapid System 2.0 uses 3 proven technologies membrane filtration, ATP bioluminescence, and image analysis for the early detection of viable microorganisms while leaving microorganisms intact for later identification.
Section Overview
- Regulatory Compliance for the Milliflex® Rapid Method
- One System, Two Critical Tests (Bioburden and Sterility Testing) with Milliflex® Rapid 2.0
- Advantages of the Dual-Purpose Milliflex® Rapid System 2.0
- Benefits of Rapid Microbial Detection System
- Familiar Microbial Testing Workflow, Proven Technologies- Milliflex® Rapid System 2.0
- Services for Milliflex® Rapid System
- Related Products
Regulatory Compliance for the Milliflex® Rapid Method
The United States Pharmacopeia (USP) recently approved updates to USP <73>: ATP Bioluminescence-Based Microbiological Methods specifically designed for detecting microbial contamination in short shelf-life pharmaceutical products.
This chapter facilitates a risk-based approach to sterility testing, making it particularly relevant for products that require immediate administration. It aligns with these guidelines by efficiently measuring adenosine triphosphate (ATP) to indicate viable microorganisms, resulting in quicker test results than traditional methods.
More specifically, USP <73> streamlines the user validation process, significantly reducing implementation timelines for systems such as Milliflex® Rapid 2.0 by allowing the user to leverage critical vendor generated validation data (ie LOD, equivalency) within their Primary Validation Package (without having to re-execute on-site).
This new Chapter enhances previous Guidance USP <1071> which offers a risk-based approach to using RMMs for detecting contamination in short-life products, emphasizing validation, proficiency testing, and updated technologies
Together USP <73>, USP <1071>, USP <1223>, Ph. Eur. 5.1.6, PDA TR33, Quality by Design provide complementary frameworks for the adoption and implementation of Rapid Microbiological Methods (RMMs).
The ATP bioluminescence technology used by the Milliflex® Rapid System 2.0 is recognized as an alternative detection method by:
- FDA1,2
- PDA Technical Report No. 33: Evaluation, Validation and Implementation of New Microbiological Testing Methods3
Membrane filtration is a methodology recommended by US, European, and Japanese standards to capture microorganisms. The Milliflex® Rapid 2.0 system is calibrated to International light standards [LNE/NIST] and meets electrical conformity to the CE mark.
21 CFR Part 11 Compliance Ready
The Milliflex® Rapid 2.0 software meets the requirements of FDA Regulation 21 CFR Part 11 for Electronic Records. The software’s powerful batch reporting feature includes capabilities for electronic signing, performing audit trails, and preventing the alteration of data files. Access and editorial privileges are controlled via the software administrator module, ensuring secure data acquisition and audit trails conforming to 21 CFR Part 11. Download the software here.
One System, Two Critical Tests: Bioburden and Sterility Testing
Milliflex® Rapid System 2.0 for Sterility Testing
The Milliflex® Rapid System 2.0 is an automated solution for the rapid detection, imaging, and quantification of viable microbial contaminants in filterable samples throughout the manufacturing process. The system's results will help to improve process control, product yield, and the timely release of final products. In case of contamination, corrective action can be taken earlier, avoiding loss of time, money, and production capacity.
Milliflex® Rapid System 2.0 for Bioburden Testing
The Milliflex® Rapid System 2.0 offers an efficient solution for rapid bioburden testing, enabling quick detection and enumeration of viable microorganisms in filterable samples across various stages of the manufacturing process. By delivering results in just a quarter of the time required by traditional methods, this system significantly enhances in-process control and final product quality assurance. The ability to obtain faster bioburden results allows for more timely interventions, optimizing production workflows and reducing the risk of costly contamination-related delays or product losses.
Advantages of the Dual-Purpose Milliflex® Rapid System 2.0
- Versatility: Suitable for both sterility and bioburden testing by changing media only
- Time-saving: Significantly speeds up sterility and bioburden testing results
- Cost-effective: One system for multiple applications. Lower overall investment compared to separate systems
- Minimal training required for dual functionality
- Aligned with pharmacopeia standards for both test types
Benefits of Rapid Microbial Detection System
The Milliflex® Rapid System 2.0 offers significant advantages for microbial contamination testing:
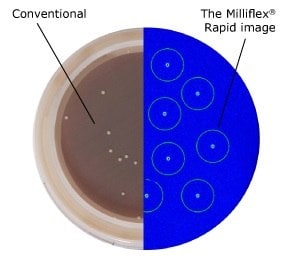
Figure 1.Conventional after 6 days (left) vs. Milliflex® Rapid after 2 days (right) method: C. acnes type III on Schaeddler Blood Agar, 35 °C.
- Consistently fast and reproducible results
- 75% faster - detection of microbial contamination in just 5 days, compared to 14 days for traditional methods
- Sensitivity down to 1 CFU per sample
- Correlation of CFU counts with those of traditional methods
- Data integrity thanks to 21 CFR Part 11 compliant software
- Easy handling and validation
Milliflex® Rapid System Applications
The Milliflex® Rapid System 2.0 is the perfect rapid solution for bioburden and sterility testing needs. It is compatible with a wide range of filterable samples from the pharmaceutical, beverage, and personal care industries:
- Raw materials
- Sterile and non-sterile final products
- Water samples
- Mineral water and beverages such as iced tea, flavored water, beer, and wine
- Cell-based matrices*
*Consult our application scientists for supporting protocols.
Familiar Microbial Testing Workflow, Proven Technologies
Recognized as an alternative detection method by FDA and PDA, compliant with US, European, and Japanese standards for membrane filtration, the Milliflex® Rapid System 2.0 is based on three proven technologies, which makes validation easier.
Without disrupting your process, the Milliflex® Rapid System 2.0 can significantly speed up your sterility and bioburden testing results. Watch the video to learn the many benefits of this automated system along with step-by-step workflow for rapid microbial detection.
The complete workflow for microbial testing including sample preparation, microbial identification, and enumeration of microcolonies is given below, along with the 3 proven technologies: membrane filtration, ATP bioluminescence, and image analysis.

Step 2: Application of Bioluminescent Reagents
Place the membrane filter on the Milliflex® Rapid 2.0 AutoSpray Station for the application of reagents. The Milliflex® Rapid 2.0 AutoSpray Station automatically applies the two reagents across the membrane surface in order to reveal the ATP present in all living microorganisms.
Proven technology - ATP Bioluminescence:
Found only in living cells, ATP is a great indicator of cell viability. Unlike other methods that detect both living and dead cells, the ATP method is highly reliable and consistent with the detection rates of current compendial testing methods.

Step 3: Enumeration of Microorganisms
Transfer the membrane filter to the Milliflex® Rapid System 2.0 Detection Tower for enumeration of microcolonies. The system counts each microcolony and stores the data for downloading, printing, and retrieval. Within two minutes, the sample is analyzed, displaying results, along with the batch history.
Proven technology - Image Analysis for Enumeration of Microorganisms:
The Milliflex® Rapid 2.0 system uses a complementary metal oxide semiconductor (CMOS) camera to detect and enumerate any microcolonies. The ATP concentration required for recognition is equivalent to one yeast or mold cell or approximately 100 bacterial cells, depending on their metabolic state. The camera’s sensitivity, combined with state-of-the-art image analysis and optimized reagents, requires only a short incubation period to generate enough ATP for the detection and enumeration of microcolonies.

We provide technical support and a comprehensive service to help you save your QC resources and facilitate the implementation of your Milliflex® Rapid System 2.0 into your daily testing routine, with our service offerings:
Evaluation Phase
- Feasibility studies for your specific samples
Implementation Phase
- Method Development at your site or remote
- Ready-to-use, GMP-compliant validation protocols
- Validation services: ready to use GMP & digital protocols, IQOQ execution service, PQ consultancy
- Advanced operator training
Routine use Phase
- Preventative maintenance
- Repair coverage
- Calibration source management
To learn more about our services or to request information, please contact our support team.
References
To continue reading please sign in or create an account.
Don't Have An Account?