Development of a Novel Cell Culture Medium for AAV Production With Multiple HEK293 Lineages and Process Optimization
Adeno-associated virus (AAV) has become a prominent vector to treat life-threatening genetic diseases. As the demand for AAV therapeutics increases, so does the need for large quantities of high titer AAV. A versatile GMP medium developed for AAV production and process optimization is vital to achieving the high AAV titers essential to support this rapidly growing industry.
Additionally, the Gene Therapy industry relies on a wide variety of commercially available HEK293 cell lines for AAV production, each with unique properties, and finding the optimal medium to use is critical for achieving cell growth and AAV titer goals but can be time consuming.
Having a single formulation that matches the nutritional requirements of multiple HEK293 lineages could save time in process development and optimization. This medium should target maximizing viable cell density (VCD) and AAV titer, while also enabling transient transfection. Until AAV production moves to stable producer HEK293 cell lines, the current practice for AAV production relies on plasmids and transfection reagents for transient transfection. However, specific raw materials found in many cell culture media formulations, such as iron citrate, are known to interfere with common transfection reagents like PEI and must be removed or reduced from media to enable efficient transfection.
This whitepaper describes the development of the Cellvento® 4HEK Medium, a novel cell culture medium for AAV production with multiple HEK293 lineages. To maximize AAV titers across multiple HEK293 cell lines, the medium development process was performed with three different HEK293 cell lines, each one with diverse growth rates. Then, the medium prototype was optimized to provide the highest AAV titer across the different cell lines. Following media development, we have sought to optimize the PEI transfection and bioreactor parameters. With this approach, the highest VCD and AAV titers can be achieved without the bottleneck of a non-optimized step in the AAV production process.
Results
Media Development Process
Cellvento® 4HEK chemically defined cell culture medium was developed using our proprietary media development process with the goal of maximizing viable cell density and AAV titer via PEI transfections in multiple HEK293 cell lineages.
In order to maximize AAV titers across multiple HEK293 cell lines, media development for AAV production was performed with three different HEK293 cell lines, each one with diverse growth and AAV production rates. As presented in Figure 1, media development began with a design of experiments (DoE) media screen for growth and AAV2 productivity. Briefly, each of the three HEK293 cell lines was adapted into 40 different media prototypes to determine the formulation with the highest performance across all three cell lines. Then, data from the media screen was analyzed with a Multivariate Data Analysis (MVDA) to identify critical components for growth and AAV production. These components were then optimized through multiple rounds of factorial DoE. Due to the complexity of PEI-based transfections, optimization of certain critical raw components required a balance between supporting high cell growth and preventing inhibition of the transfection process. Because the transfection process (with or without dilution prior to transfection) typically occurs on passage day three, medium was optimized to support robust growth early in the growth curve for HEK293 cell lineages, rather than the common method of optimizing the medium for maximum peak viable cell density later in the growth curve. Following the prototype optimization, the final formulation was defined. As shown in Figure 1, the media optimization steps enabled improvement to growth performances and importantly increase of AAV titer. Once the final formulation of the optimized prototype was confirmed, the medium was streamlined, stability tested and named: Cellvento® 4HEK Medium.
Figure 1A.Process of developing media for multiple HEK293 lineages. Design of Experiment (DoE) designed media screens for growth and AAV productivity.
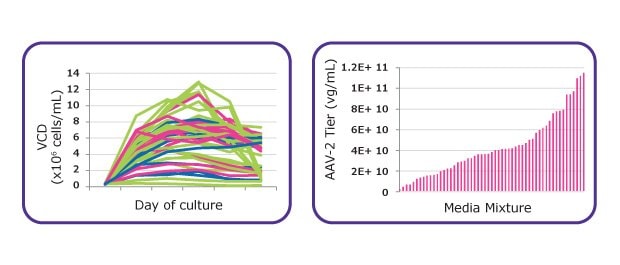
Figure 1B.Multivariate Data Analysis (MVDA) used to identify critical components.
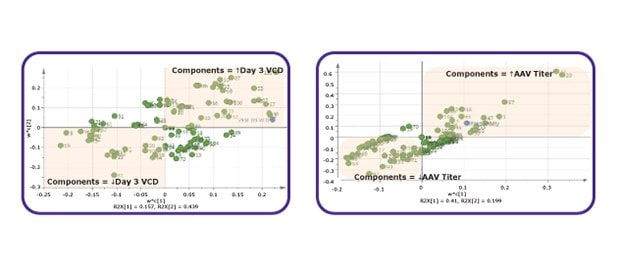
Figure 1C.DoE experiments for optimization of critical components.
Figure 1D.Process of developing media for multiple HEK293 lineages. Optimization and final formulation.
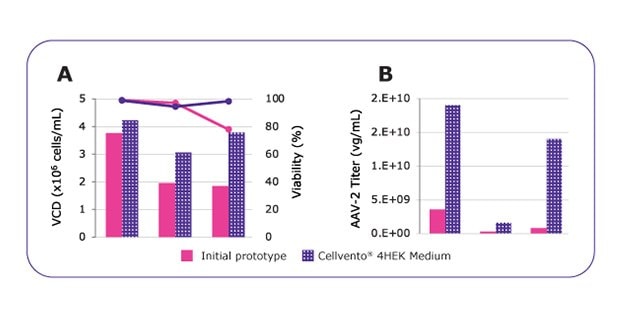
Figure 2.MVDA analysis enabled to highly improve media performances. Growth performances (A) and AAV2 productivity (B) comparison between the initial medium prototype and the optimized and final formulation named Cellvento® 4HEK medium. *Vendor recommends DNA:PEI ratio of 1:4, however, due to toxicity (data not shown), the DNA:PEI ratio was reduced to 1:3 in this experiment.
Performance Evaluation
To assess the improved cell growth and increased AAV2 titer with Cellvento® 4HEK Medium, its performance was evaluated against three major competitor formulations in four HEK293 cell lines. As shown in Figure 3A, we compared the day three growth of the four HEK293 cell lines and found that Cellvento® 4HEK Medium is the best medium to support rapid growth of all cell lines evaluated. The second-best medium was the EX-CELL® CD HEK293 Viral Vector Medium that supports the growth of all cell lines. We observed that the HEK293-3 cell line, which did not grow at all in Competitor A’s medium and displayed lower viable cell density and viability in Competitor B’s and Competitor C’s formulations. While Competitor B’s cell culture medium supported growth in all four cell lines, it inhibited transfection (Figure 3B), thus significantly reducing the AAV2 titer (Figure 3C).
As demonstrated in Figure 3, Cellvento® 4HEK Medium can support higher viable cell density on Day 3 as well as higher AAV2 titer compared to competitor media tested.
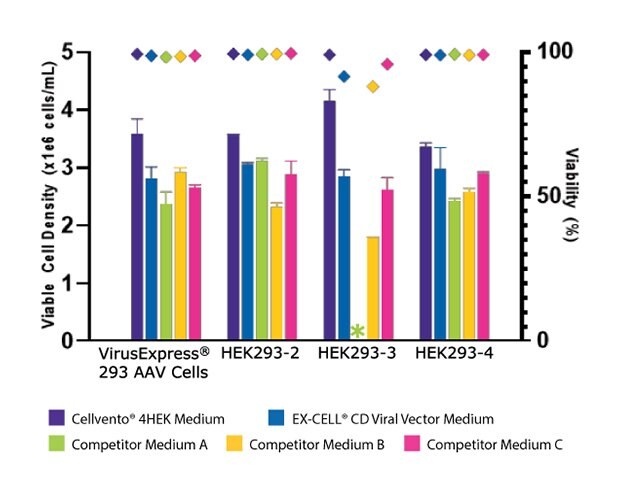
Figure 3A.Cellvento® 4HEK Medium supports higher VCD and AAV2 titer than other media tested. Day three viable cell density (VCD) and viability of four HEK293 cell lines cultured in Cellvento® 4HEK Medium and three competitor formulations. HEK293-3 cell line would not adapt to Competitor A’s formulation. For all cell lines, the highest VCD and viability were reached using Cellvento® 4HEK as growth medium.
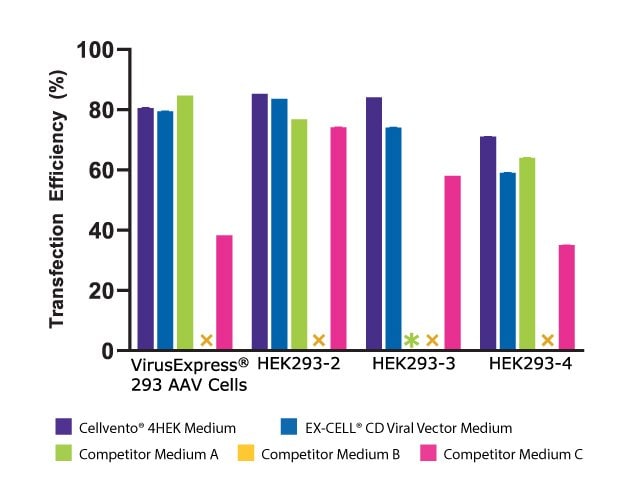
Figure 3B.Bar plot showing the AAV2 titer (GC/mL) achieved in four cell lines, each transfected in Cellvento® 4HEK and three competitor formulations. For all cell lines, the highest AAV2 titer was reached using Cellvento® 4HEK as growth medium, complexation reagent, and production medium (Cellvento® 4HEK was designed to use through the entire transfection process).
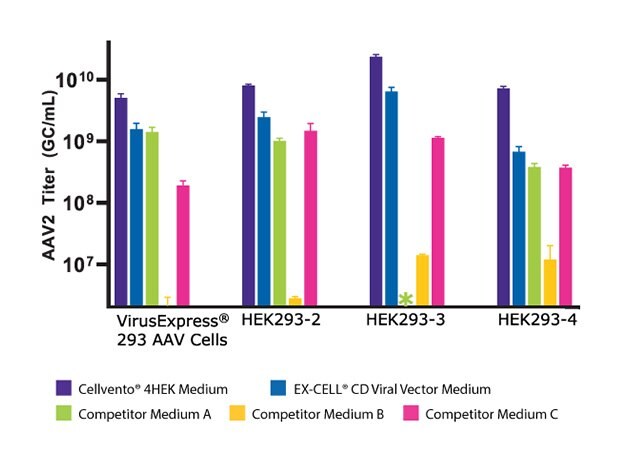
Figure 3C.For all cell lines, the highest AAV2 titer was reached using Cellvento® 4HEK as growth medium, complexation reagent, and production medium (Cellvento® 4HEK was designed to use through the entire transfection process).
Transfection Optimization
Optimization of the transfection process is paramount to achieving the high viral titers required for gene therapies. Triple plasmid transfection is a complex process with a seemingly endless number of parameters to consider for optimization. Because of this, and after having developed a medium providing a high AAV titer, we focused on optimizing our transfection processes to further increase AAV titer produced with the VirusExpress® AAV Production Cells and the HEK293-3 cell line.
The first step of the transfection optimization was to evaluate the impact on the transfection reagent used. We evaluated two PEI transfection reagents, PEIpro® (PolyPlus) and PEI Max® (Polysciences). We found that PEIpro® provided better performances for the VirusExpress® 293 AAV cell line whereas PEI Max® worked best for the HEK293-3 cell line (data not shown). Then, both processes required further optimization beyond the vendor recommended transfection method. As demonstrated in Table 1 and Figure 4, we were able to improve over 10x the AAV titer by optimizing the transfection protocols. This experiment demonstrated that it’s essential to optimize all transfection parameters for each cell line.
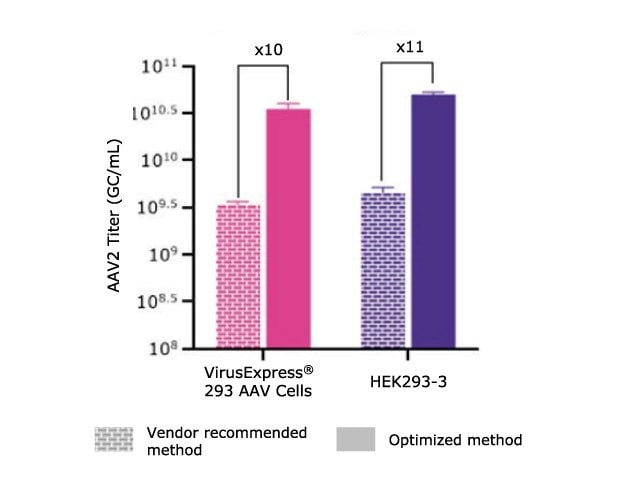
Figure 4.AAV2 titer (GC/mL) achieved using vendor-recommended transfection methods compared to optimized transfection methods for the VirusExpress® 293 AAV and HEK293-3 cell lines. Greater than 10-fold increase in AAV2 titer achieved post-optimization. *Vendor recommends DNA:PEI ratio of 1:4, however, due to toxicity (data not shown), the DNA:PEI ratio was reduced to 1:3 in this experiment.
Bioreactor Culture Optimization
Once our transfection methods were optimized for AAV2 production, we began optimizing the scale-up process, beginning at the microbioreactor scale (15 mL). As with transfection optimization, bioreactor parameters should be evaluated and optimized for each cell line, basal medium and transfection cocktail combination. In addition, bioreactor parameters (pH, %DO, and agitation rate) for cell growth and AAV production may have different settings and should be evaluated separately. Therefore, the microbioreactor process was broken down into three phases for optimization: cell growth, AAV production, and finally the full run including growth, transfection, and AAV production all within the microbioreactor vessels.
For the HEK293-3 cells, our study demonstrated that achieving peak day three growth required high agitation rates (1,400 rpm) and higher pH (7.2-7.4) during the growth phase; whereas the agitation rate had to be slowed (700 rpm) and the pH reduced (6.8-7.0) for optimal AAV production in the microreactors. Similar parameters were determined for the VirusExpress® 293 AAV cell line.
As presented in Table 2 and Figure 5, after successful optimization of the cell culture medium, transfection process, and microbioreactor processes, we were able to get an AAV2 titer of 4.7x1010 GC/mL with the HEK293-3 and 1.2x1010 GC/mL with the VirusExpress® AAV cells.
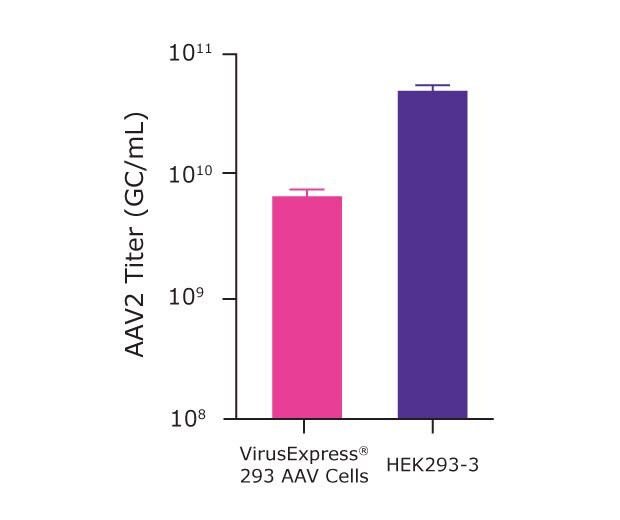
Figure 5.AAV2 titer achieved using optimized Ambr® 15 parameters for transfection of VirusExpress® 293 AAV cells and HEK293-3 cells.
Finally, following optimization of the microbioreactor parameters, we were ready to further scale-up our process to the benchtop bioreactor level. Although the microbioreactor system is commonly used as a scale-down model for larger bioreactors, due to certain aspects of the process (vessel shape, gas exchange and agitation methods, surface: volume ratio, etc.), further optimization of agitation and %DO may be required when moving to larger scale bioreactors. This is why we optimized the process parameters for the Mobius® 3L cell culture. As shown in Table 3 and Figure 6B our data demonstrated that the VirusExpress® AAV cells, cultivated with the Cellvento® 4HEK Medium and in 3L bioreactor produced an AAV2 titer of 4.7x1010 GC/mL.

Figure 6B.Mobius® 3L Bioreactor
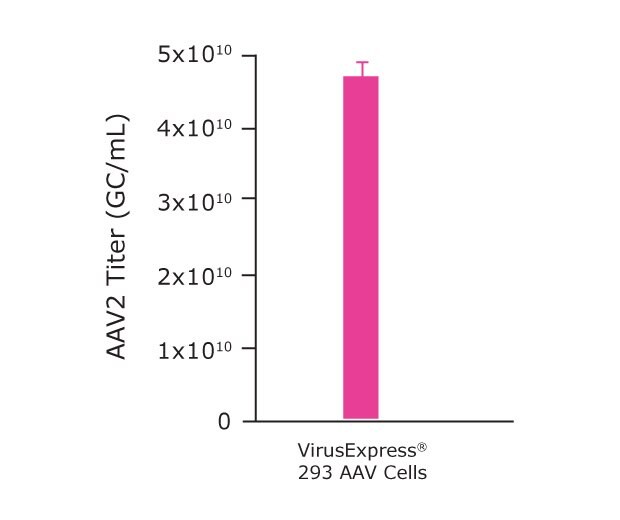
Figure 6B.Mobius® 3L parameters optimization for AAV2 production. AAV2 titer achieved using an optimized Mobius® 3L Bioreactor.
Conclusions
Finding the optimal cell culture medium formulation for AAV production with HEK293 cells, especially with transient transfection process, can be time-consuming and is essential for maximizing AAV titers. There is a wide variety of HEK293 cell lines with diverse nutritional requirements and transient transfection has a multitude of interacting variables. Because of this, the Cellvento® 4HEK Medium has been specifically developed to support the growth of multiple HEK293 cell lineages and optimized to support the entire transfection process from scale-up through production.
This whitepaper describes the Cellvento® 4HEK Medium development process and then presents the optimization of the transfection process and cell culture parameters from microbioreactor to 3L bioreactor. Regarding any media evaluation, the main recommendation is to fully adapt cells into new formulations with a minimum of three adaptation passages. Direct adaptation is possible; however, some cell lines may require serial dilutions to properly adapt to a new medium formulation. The transfection optimization study demonstrates that vendor-recommended methods need to be optimized, especially the DNA:PEI ratio, the pDNA:cell ratio, as well as the transfection cell density, to get the highest AAV titer. Finally, for the cell culture parameters optimization, our analysis demonstrates that it’s important to evaluate separately cell growth and AAV production phases for which we found different optimal pH, %DO and agitation rate.
Overall, this whitepaper demonstrates that achieving high AAV titer requires optimization of each step of the production process, beginning by using a high-performing cell culture medium and then optimizing the transfection process and the cell culture parameters.
Materials and Methods
Cell Lines and Cell Culture Media
HEK293 cell lines: VirusExpress® 293 AAV Production Cells (SAFC, VP002), and 3 other HEK293 cell lines (named in this study HEK293-2, HEK293-3 and HEK293-4). HEK293 cell lines were adapted to all tested media for a minimum of 3 passages prior to growth and production assays.
Cell Culture Media: Cellvento® 4HEK Medium (SAFC, 1251931000), EX-CELL® CD HEK293 Viral Vector Medium (SAFC, 14385C-1000mL) and 3 chemically defined competitor media (named in this study Competitor A, Competitor B, and Competitor C). All media were supplemented with 6-8 mM L-Glutamine (Sigma-Aldrich, G7513).
Cell Culture
For passaging, all cells were seeded at 0.5x106 cells/mL for three-day cultures and 0.3x106 cells/mL for four-day cultures. Cultures were expanded in baffled shake flasks at 37 °C with 5% CO2 agitated at 135 RPM/25 mm throw. Baffled shake flasks help mitigate clumping. Note: Cell line vendors recommended 8% CO2, however, a comparison of 5% vs 8% CO2 showed better growth and AAV titer at 5% CO2 (data not shown).
Growth and productivity assays were performed in TPP® TubeSpin Bioreactor tubes (MidSci, TP87050) at 37 °C with 5% CO2 agitated at 295-315 RPM/25 mm throw. Cell counts were performed on the ViCell XR analyzer (Beckman-Coulter).
Transfection
At 24 hours post-dilution, HEK293 cultures were triple plasmid transfected in TPP® TubeSpin Bioreactor tubes (MidSci, TP87050) or Ambr® 15 microbioreactors to produce AAV2 encoding GFP.
For media development and benchmarking assay, HEK293 cultures were transfected at 2x106 cells/mL with a total of 0.5 µg plasmid DNA/1 x106 viable cells using PEI MAX® transfection reagent (Polysciences, 24765) at a DNA:PEI ratio of 1:2. DNA and PEI were complexed in media screen mixtures or Cellvento® 4HEK Medium for 15 minutes at 10% of the culture volume.
For media development, AAV2 plasmids consisted of pHelper, pAAV-RC2, and pAAV-GFP (VKP402, CellBiolabs) at 2:1.6:1 ratio. For transfection optimization, AAV2 plasmids consisted of pALD-HELP, pALD-Rep/Cap2, and pALD-ITR GFP (Aldevron). Plasmids were changed for internal alignment.
Cultures were harvested 72 hours post-transfection.
Transfection efficiency was determined by quantifying the percent GFP positive cells by flow cytometry using a NovoCyte® 3005 Flow Cytometer (Agilent).
AAV Titer Quantification by ddPCR
Harvested cultures were lysed with a solution of 0.5% Tween-20, 2mM MgCl2, and 100 U/mL Benzonase® endonuclease (SAFC, E1014) followed by the addition of NaCl for a final concentration of 0.5M. Lysates were treated with DNase (MP Biomedicals, 190062), diluted in DNase Buffer (ThermoFisher, B43), then the reaction was quenched with EDTA (Teknova, E0307). Samples were then diluted 1:150 in DEPC-treated water (ThermoFisher, AM9906) and heat-lysed at 95 °C for 10 minutes. The supermix reaction was set-up using primers and a FAM-labelled probe targeting GFP. Droplets were generated with the Bio-Rad Droplet Generator (Bio-Rad), then transferred to the thermal-cycling plate and heat-sealed with pierceable foil. The final reaction plate was then placed in the C1000 Touch™ Thermal Cycler (Bio-Rad) and PCR was completed with the cycle parameters described in Table 5. After PCR, the final reaction plate was placed in the QX100 Droplet Reader (Bio-Rad) and the droplets were analyzed following manufacturer’s recommendations.
Microbioreactor Cell Culture
Growth: Media was conditioned in vessels ≥24 hours prior to cell inoculation in Ambr®15 Cell Culture Microbioreactor System (Sartorius Stedim). Vessels were seeded at 0.5x106 viable cells/mL. Cell growth and viability were monitored daily on ViCell XR analyzer (Beckman-Coulter) for up to four days, at which time, the run was concluded.
Production: Cells were diluted in flasks to 1x106 viable cells/mL 24 hours prior to transfection. Cells were transfected in flasks using PEI (see Cell Transfection for AAV2 Production) and then immediately transferred to Ambr® vessels via Ambr® liquid handler. Cell growth, viability, transfection efficiency, and virus production were monitored on ViCell XR, Novocyte (Agilent) and ddPCR (Bio-Rad) post transfection. Cells and virus were harvested three days post transfection.
Growth, Transfection, and Production Run: Vessels were seeded at 1.7x106 viable cells/mL 24 hours prior to transfection. Cells were transfected manually or via Ambr® liquid handler using PEI. Cell growth, viability, metabolites, transfection efficiency, and virus production were monitored post-transfection. Cells and virus were harvested three days post-transfection.
Related Products
To continue reading please sign in or create an account.
Don't Have An Account?