Anti-FLAG® M2 Magnetic Beads
- Anti-FLAG® M2 Magnetic Beads for FLAG® Tag Protein Capture
- Sample Preparation and Affinity Purification
- Electrophoresis and Western Blotting
- Comparison of Elution Techniques for FLAG-tagged Fusion Proteins
- FLAG® Tag Antibodies, Control Proteins, and Affinity Purification Tools
Anti-FLAG® M2 Magnetic Beads for FLAG® Tag Protein Capture
Anti-FLAG® M2 magnetic beads provide an easy, fast and convenient method for the detection and capture of fusion proteins with the FLAG® peptide sequence. Beads are composed of a murine derived, anti-FLAG® clone M2 monoclonal antibody attached to superparamagnetic iron impregnated, 4% agarose beads. The anti-FLAG® M2 antibody recognizes the FLAG® octapeptide sequence (N-Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys-C) at the N-terminus, Met-N-terminus, or C-terminus locations of a fusion protein expressed in mammalian and bacterial cells.
There are different options for the elution of a fusion protein when using anti-FLAG® M2 magnetic beads, which include peptide competition, low pH, and SDS-PAGE sample buffer. Here we compared the three elution methods for control fusion protein N-terminal Met-3XFLAG®-BAP (P7582) or C-terminal FLAG®-BAP (P7457) incubated alone or spiked into an A431 cell lysate.
Elution by competition with 3X FLAG® peptide (F4799) as well as acidic pH (2.5-3.0) provided the most efficient elution conditions for the Anti-FLAG® M2 magnetic beads. When eluting with 3X FLAG® peptide, samples required a 30-minute incubation with a solution of 100-150 ng/µL FLAG peptide, while for pH elution, samples required less than a 10-minute incubation with 0.1 M Glycine HCl pH 2.5-3.0. We also demonstrated that elution by high pH (8-10) was not sufficient with this type of beads, and that extended incubations with glycine HCl do not increase the amount of protein eluted. Finally, we also compared samples eluted using SDS-PAGE sample buffer. This elution condition was simple, but since the M2 antibody is denatured by the presence of SDS in the loading buffer, analysis by gel electrophoresis showed bands corresponding to the heavy and light chains of the anti-FLAG® antibody.
Sample Preparation and Affinity Purification
Anti-FLAG® M2 magnetic beads (M8823) were thoroughly resuspended and 20 µL of suspension (including 10 µL of packed resin) were aliquoted into several 1.5 mL microcentrifuge tubes. Tubes were placed in a PureProteome™ Magnetic Stand (LSKMAGS08), and supernatant was removed and discarded after all beads had migrated to the magnet. Resin was equilibrated by adding 200 µL of Tris Buffer Saline (TBS) followed by sample incubation (Figure 1).
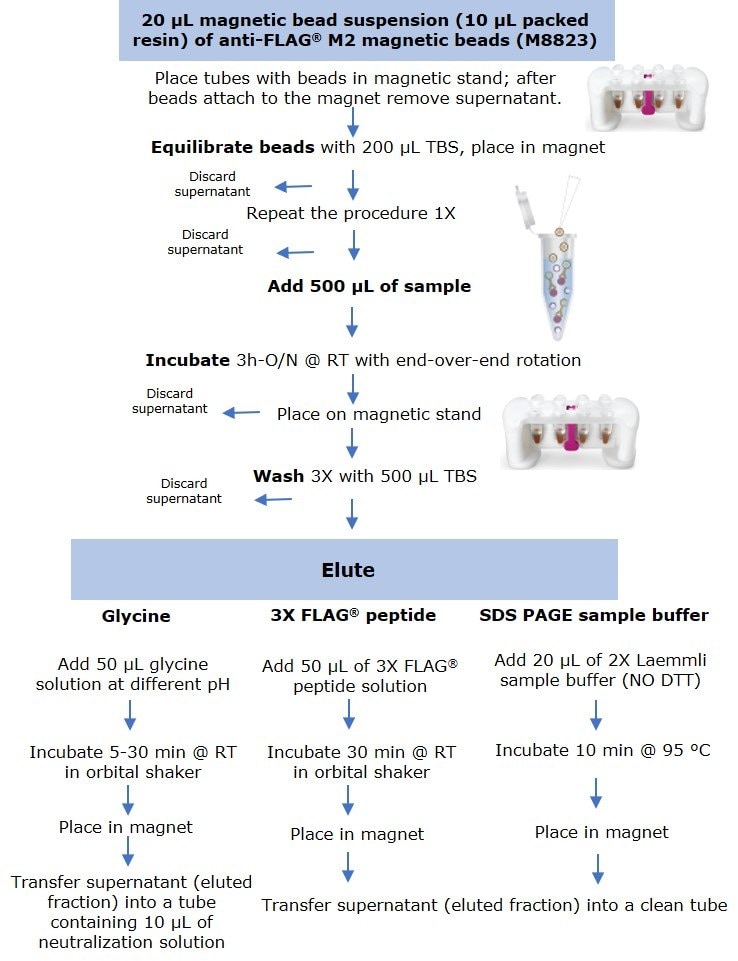
Figure 1.Protocol for small scale immunoprecipitation by anti-FLAG® M2 magnetic beads using three elution methods.
Samples were prepared by diluting the C-terminal FLAG-BAP™ fusion protein or the N-terminal FLAG-BAP™ fusion protein to a final concentration of 0.6 ng/µL (300 ng protein in 500 µL sample). FLAG-BAP™ fusion protein was also spiked at the same final concentration into an A431 cell lysate diluted to a total protein lysate concentration of 1.6 ng/µL. To maintain the beads in suspension and in contact with 500 µL of sample, tubes were incubated for 3 hours to overnight using an end-over end rotor. After incubation, all tubes were placed into the magnet stand and the supernatant was removed, followed by three washes with 500 µL of TBS. Protein was eluted using three methods:
- Elution by competition using the 3X FLAG® peptide was performed at room temperature by incubating the beads for 30 min with 50 µL of 100 ng/µL peptide solution using an orbital shaker.
- For samples eluted with SDS-PAGE sample buffer, 20 µL of 2X Laemmli sample buffer (without DTT) was added, and tubes were boiled for 10 min at 95°C, before loading onto a gel.
- Elution by pH was performed at room temperature by incubating the beads for 5 to 30 min (depending on the experiment) with 50 µL of glycine HCl (pH 2.5-3.0) or glycine NaOH (pH 8.0 – 10) using an orbital shaker.
After incubation, all tubes were placed into the magnetic rack and the supernatant (eluted fraction) was transferred to a clean microcentrifuge tube. Samples eluted with low pH were immediately neutralized by adding 10 µL of 0.5 M Tris, 1.5 M NaCl pH 8.0 solution.
Electrophoresis and Western Blotting
Samples were separated using a 4-12% SDS-PAGE gels, run for 30 minutes at 200 volts. Gels were stained with Coomassie or transferred to an Immobilon®-P PVDF membrane. After transfer, blots were blocked with 2% Non-Fat Dry Milk (NFDM) for 1 hour, followed by 1-hour incubation with anti-FLAG® M2 antibody (F3165) diluted 1:1000 in blocking buffer. Blots were washed three times in TBS-T and incubated for 1 hour with goat anti-mouse IgG HRP (AP124P) diluted 1:50,000.
For detection, all the blots were incubated for 5 min with Immobilon® Forte Western HRP substrate and image was acquired using a digital imaging system.
Comparison of Elution Techniques for FLAG-tagged Fusion Proteins
Two control fusion proteins, N-terminal FLAG-BAP™ and C-terminal FLAG-BAP™, as well as an A431 cell lysate spiked with N-terminal FLAG-BAP™, were incubated with anti-FLAG® M2 magnetic beads and eluted using the three different elution methods. Figure 2 shows that proteins were efficiently eluted using all three methods, with extra bands corresponding to the anti-FLAG® M2 antibody’s heavy and light chain visible when SDS loading buffer was used as an eluent.

Figure 2.Comparison of the three methods of elution used with anti-FLAG® M2 magnetic beads. St=starting material; 3X=3X FLAG® peptide, pH=2.5 glycine
Glycine HCl (pH 2.5-3.0) and glycine NaOH (pH 8-10.6) solution were used to elute eight samples containing A431 cell lysate spiked with N-terminal FLAG-BAP™ control protein. Eluted fractions were separated by electrophoresis and electroblotted. Results indicated that anti-FLAG® M2 Magnetic Beads can only be eluted under acidic conditions (Figure 3).

Figure 3.Elution by a wide range of pH (2.5 to 10.6), compared with elution using 3X FLAG® peptide. Left: Coomassie-stained gel. Right: Immunoblot with anti-FLAG® M2 antibody.
To demonstrate that elution by pH does not require more than 10 minutes of incubation with the beads, different samples were subjected to different incubation times using 0.1 M glycine HCl pH 2.5, 2.8 and 3.0. Figure 4 shows that yield was comparable independent of incubation time, and similar to elution by competition with 3X-FLAG peptide.
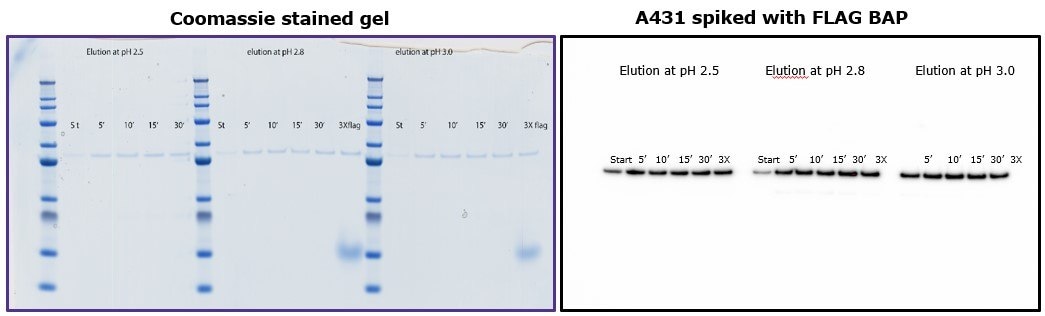
Figure 4.Comparison of eluted fractions after incubation of 5 to 30 minutes with glycine. Left: Coomassie-stained gel. Right: Immunoblot with anti-FLAG® M2 antibody.
FLAG® Tag Antibodies, Control Proteins, and Affinity Purification Tools
To continue reading please sign in or create an account.
Don't Have An Account?