Capto Adhere Anion Exchanger for MAbs & Bind/Elute
Capto adhere is a multimodal strong anion exchanger for BioProcess applications. It was originally designed for post-protein A purification of MAbs at process scale in flowthrough mode. However, Capto adhere can also be used in bind/elute mode and for applications other than MAbs.
The strong multimodal ion exchange ligand (Figure 3.2) gives a different selectivity compared with traditional ion exchangers. Capto adhere can remove key impurities in a single step, allowing the design of a two-step process together with a protein A media (e.g., MabSelect™, MabSelect SuRe™, or MabSelect SuRe LX). Capto adhere can also be used in combination with AIEX or CIEX for polishing, as a second or third step in any MAb purification platform (Figure A3.1 in Appendix 3, Use of multimodal media for MAb purifcation).
Key performance benefits of Capto adhere include:
- high load and productivity
- impurity removal to formulation levels in post-protein A purification. Removal of:
- antibody dimers and aggregates
- HCP
- nucleic acids
- viruses
- leached protein A
- endotoxin
- wide operational window of pH and conductivity
- savings in time and operating costs with a two-step chromatographic process
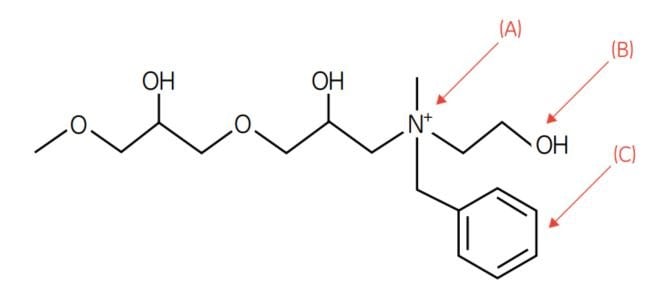
Figure 3.2.The design of the Capto adhere ligand, N-benzyl-N-methyl ethanolamine. This ligand exhibits several possibilities for interaction with proteins. The most pronounced are electrostatic interaction, hydrogen bonding, and hydrophobic interaction, as shown by arrows: (A) for electrostatic interactions; (B) for hydrogen bonding; and (C) for hydrophobic interactions.
As a first option, Capto adhere is recommended to be operated in flowthrough mode, because this provides higher throughput. In flowthrough mode, the antibodies pass directly through the column while contaminants (leached protein A, aggregates, HCP, nucleic acids, and viruses) are adsorbed. Nevertheless, in cases where low molecular weight impurities, such as antibody fragments, are present, Capto adhere in bind/elute mode might give higher purity.
pH Operating Window
As shown in Figure 3.3, the pH window of gradient elution of MAbs using Capto adhere differs from that of traditional media and should be determined for each target protein. In the experiment shown, five different antibodies with varying pI were run in bind/elute mode on Capto adhere and Capto™ Q (see the discussion of bind/elute vs flowthrough mode in Chapter 2).
Analytical loads of the antibodies were used, and they were eluted from the chromatography media with a pH gradient. The antibodies elute in the same order on both media, but they elute much earlier—that is, at higher pH—on Capto™ Q. This result indicates that additional interactions are involved on Capto adhere. Antibodies eluted below the isoelectric point on Capto adhere.
With respect to pH, the operating window for Capto adhere is therefore at lower pH than for traditional anion exchangers. If deamidation of the antibody is an issue, being able to run at lower pH is of course beneficial.
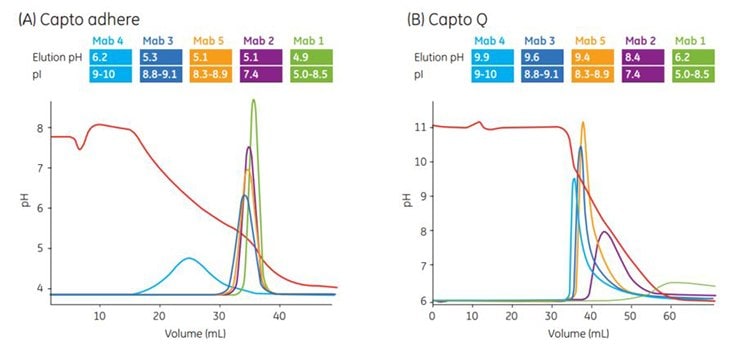
Figure 3.3.Different selectivity of Capto adhere compared with traditional ion exchangers. pH gradient elution of five MAbs on Capto adhere and Capto™ Q. For the pH gradient (A) A-buffer (equilibration buffer) was 20 mM Na-citrate + 20 mM Na-phosphate, pH 7.8, and B-buffer was buffer A at pH 4.0. (B) A-buffer was 20 mM Na-phosphate, 20 mM Tris, 20 mM glycine, pH 11, and B-buffer was buffer A at pH 6.2 Gradient: 0 to 100% B, 10 column volumes (CV).
Removal of Aggregates
High antibody titers tend to increase the generation of aggregates and impurities in the feedstock. Capto adhere allows removal of aggregates to target values acceptable for formulation. To achieve the best performance with Capto adhere operated in flowthrough mode (i.e., to maximize the amount of impurities adsorbed to the medium while the monomeric MAbs pass through the column), screening for optimal loading conditions is needed. Optimization is preferably done with DoE. For details about how to set up a DoE, see Chapter 4, which includes an application example showing how Capto adhere effectively removes aggregates (Optimization of loading conditions on Capto adhere using DoE). In this work, the sample was a cell culture supernatant (CSS) containing IgG1 that was first purified on MabSelect SuRe. See also application notes 28-9078-89, “Optimization of loading conditions on Capto adhere using design of experiments” and 28-9509-60, “High-throughput screening and optimization of a multimodal polishing step in a monoclonal antibody purification process.”
Viral Clearance
An example of the use of Capto adhere for viral clearance is presented in Chapter 4 (Viral clearance using Capto adhere). In this work, Capto adhere was tested with two representative model viruses, and it was found that even at high conductivity, where traditional ion exchangers do not work, the log reduction factor was significant.
Removal of Other Impurities and Contaminants
Removal of HCP and leached protein A is illustrated in Chapter 4 (HTS and optimization of a multimodal polishing step in a MAb purifcation process using Capto adhere). Negatively charged impurities/contaminants such as nucleic acids and endotoxins are also effectively removed. Salt type and additives As previously discussed, separation of monoclonal monomer and aggregates is one of the main challenges in MAb processes. In Figure 3.4, an experiment is presented in which the effect of isopropyl alcohol and urea on monomer and aggregate static binding capacity (SBC) of Capto adhere was investigated. With 20% isopropyl alcohol, the monomer capacity decreased significantly with increased ionic strength, while the aggregate capacity remained essentially unchanged. In the case of urea, the effect of ionic strength was similar for both the monomer and the aggregates, leading to a decrease in binding capacity with increase in ionic strength. At low ionic strength, the effect of urea on capacity of aggregates was minimal, while the binding capacity for monomer decreased almost two-fold. These findings can be utilized to optimize a Capto adhere step in flowthrough mode where low monomer binding and high aggregate binding is desirable.
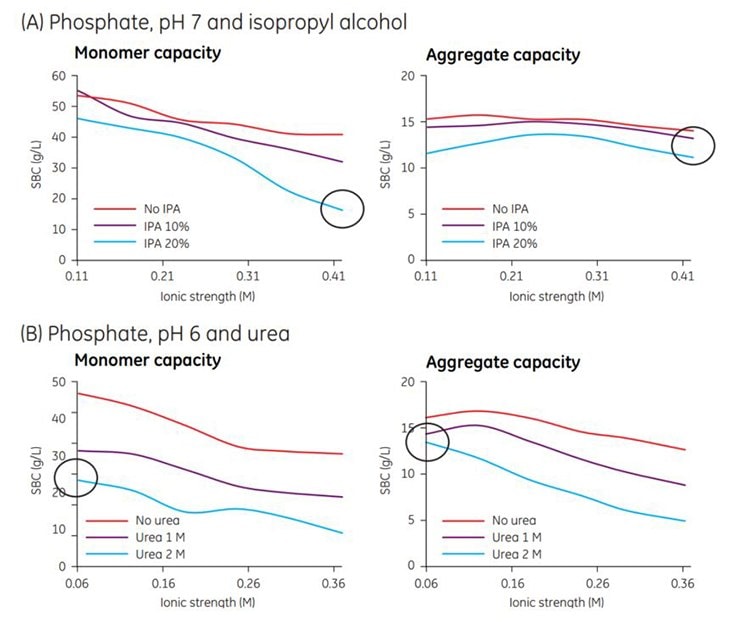
Figure. 3.4.The influence of (A) isopropyl alcohol (IPA) and (B) urea on the SBC for monomeric and aggregate forms of a MAb on Capto adhere. Phosphate was used as buffer, and ionic strength was adjusted using NaCl. At high ionic strength, isopropyl alcohol can significantly lower monomer capacity while maintaining most of the aggregate capacity (black circles). The effects are similar for urea at low ionic strength, although not identical. These results illustrate that some additives could have multiple effects, i.e., both reducing hydrophobic interactions and disrupting hydrogen bonds.
The type of salt chosen can also affect performance (Figure 3.5). In this experiment the effect of different salt species on SBC was compared. With NaI the aggregate capacity decreased significantly with increased ionic strength, while the monomer capacity was essentially unchanged. The NaI effect suggests that chaotropic salts can be used to optimize a bind/elute step, where high monomer capacity and low aggregate capacity is desirable.
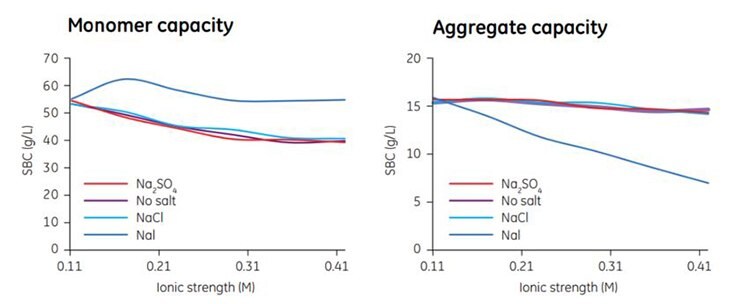
Figure 3.5.The influence of different salt types on the SBC for MAb monomers and aggregates on Capto adhere. Phosphate at pH 7.0 was used as buffer in all cases.
Regeneration
Due to its multimodal properties, regeneration of Capto adhere generally requires an acidic strip prior to CIP (see Appendix 2, Maintenance of media and storage conditions).
Materials
To continue reading please sign in or create an account.
Don't Have An Account?