Ultrapure Water for Pesticides Analysis in Cannabis Testing

Section Overview
- Cannabis Testing for Customer Safety and Compliance
- Importance of Water Quality for Analysis of Cannabis
- Study: Assessment of a Milli-Q® System for the Elimination of Pesticides From Water
- Results & Discussion: Pesticide Analysis of Pure & Ultrapure Water
- Conclusion
- Finding an Optimal Water Purification Solution
- Experimental Procedure
- Related Products
This study aimed to demonstrate the effectiveness of a Milli-Q® water purification system in removing pesticides from tap water, thereby producing ultrapure water of consistently high quality for reliable analysis of pesticides in cannabis testing. Experiments were conducted using solutions spiked with high concentrations of pesticides commonly employed in cannabis cultivation. Analysis using LC-MS/MS and GC-MS/MS revealed that none of the pesticides tested were detectable in the purified water samples. The findings indicate that the technologies integrated into the Milli-Q® system effectively eliminate these pesticide contaminants. These results underscore the suitability of Milli-Q® ultrapure water for the chromatographic analysis of pesticides and other organic compounds in cannabis testing laboratories.
Cannabis testing for customer safety and compliance
Regulatory institutions are actively developing frameworks to ensure the safety of cannabis products,1 but the national and international regulatory landscapes remain complex. In this challenging environment, testing laboratories strive to develop analytical testing methods that ensure both customer safety and compliance with regulations. Laboratories typically adhere to standards applicable to the pharmaceutical and food industries, depending on whether the cannabis products are intended for medicinal or recreational use.2
To meet local regulatory requirements, raw cannabis and cannabis-derived products undergo various tailored analyses. These analyses include testing for cannabinoid, flavonoid, and terpene profiles, as well as the detection of pesticides, solvent residues, mycotoxins, heavy metals, and microbial contaminants.3 The presence of pesticides in cannabis and its by-products can pose significant health risks, particularly for vulnerable individuals with weakened immune systems. Therefore, implementing rigorous testing protocols is essential to mitigate these health concerns.
Pesticide residue analysis has become a significant area of focus, with products tested for both authorized and unauthorized pesticides to ensure compliance with regulatory and quality standards. In North America, pesticide testing requirements vary across states and between countries. For instance, California, Oregon, and Colorado analyze a total of 66, 59, and 58 pesticides, respectively, highlighting the unique regional requirements.4 In Canada, cannabis products are screened for a total of 96 pesticides, reflecting a comprehensive approach to ensure safety and compliance.
Importance of Water Quality for Analysis of Cannabis
The analysis of pesticides in cannabis presents significant challenges due to various factors. Sample preparation is a critical phase in the workflow of pesticide analysis, influenced by the matrix effect, potential interferences, and the choice of the analytical technique, such as liquid chromatography-mass spectrometry (LC-MS) or gas chromatography-mass spectrometry (GC-MS).
Given these challenges, focusing on water quality is essential for ensuring the accuracy and reliability of pesticide analyses. The quality of water directly impacts the performance of the analytical instruments and the robustness of the results. Therefore, maintaining high standards for water quality is crucial for successful pesticide testing in cannabis products.
Study: Assessment of a Milli-Q® system for the elimination of pesticides from water
To evaluate the effectiveness of tap-to-pure and pure-to-ultrapure water systems in removing pesticides relevant to cannabis testing, two distinct challenges were conducted at different stages of the purification process of a Milli-Q® IQ 7005 water system (Figure 1). Both tap water (Pesticides injection 1) and the pure water in the tank (Pesticides injection 2) were spiked to obtain a solution of approximately 1 µg/L of 15 pesticides (TraceCERT® Multiresidue Pesticide Standard Mix 4 and Mix 6), which is about 100 times the maximum residue limit (MRL) for most pesticides. In this study, 14 of the 15 pesticides were analyzed. We chose these two standard mixes as they are suitable for use in meeting California state requirements for pesticide testing of cannabis products. The mixes contain various families of pesticide molecules, including pyrethroids, phosphates, carbamates, neonicotinoids, phthalimides, benzodioxols, organophosphorus compounds, acylalanines, and anthranilic diamides. This selection allows for comprehensive testing of pesticides commonly analyzed by cannabis testing labs, covering a range of chemical functionalities.
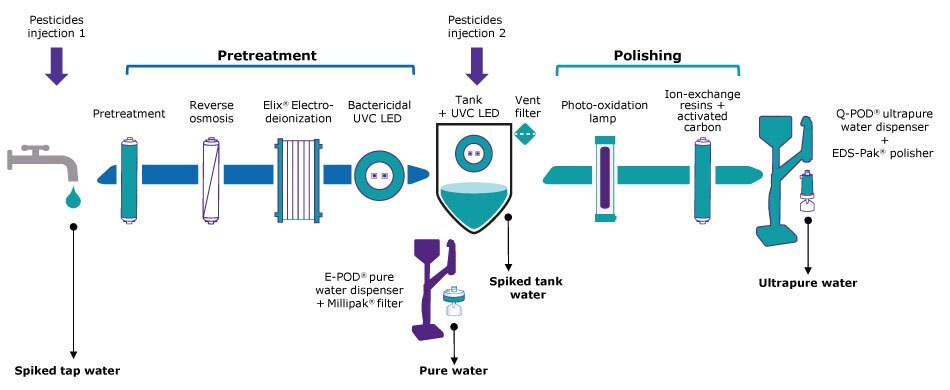
Figure 1.Experimental set-up depicting the technologies in a Milli-Q® IQ 7005 water purification system. Arrows indicate where the pesticide doping solutions were injected (in the tap water and the tank water) and the four samples that were analyzed: [(1) spiked tap feed water, (2) pure water produced after the pretreatment step, (3) spiked tank water before the polishing step, and (4) ultrapure water produced after the polishing step drawn at the point-of-dispense equipped with an EDS-PAK® polisher].
We selected to place an EDS-Pak® polisher at the Q-POD® ultrapure water point-of-dispense of the Milli-Q® IQ 7 series system. It contains specific activated carbon designed for the removal of organic contaminants behaving as endocrine disrupters.
Water samples were taken after the pretreatment and polishing steps of the water purification process and sent to a third-party laboratory for chromatographic analysis. Analyses were performed in duplicate according to methods described in the Experimental Procedure section below.
Results & Discussion: Pesticide analysis of pure & ultrapure water
Pretreatment: From tap to pure water (Type 2)
The results of LC-MS/MS and GC-MS/MS analyses showed that none of the 14 pesticides tested were quantitatively detected in pure water obtained after the pretreatment stage (Table 1 and Figure 2, left sides). The LOQ was 0.05 µg/L for each pesticide.
Similar to what was discussed in our previous study, pesticides were likely mainly captured by the activated carbon in the pretreatment cartridge. The porous nature of activated carbon creates an extensive surface area that facilitates the adsorption of molecules through intermolecular interactions, including Van der Waals forces. Furthermore, the semi-permeable reverse osmosis (RO) membrane likely played a significant role in the effective removal of remaining traces of pesticides.
Although pesticide concentrations below the LOQ were detected in pure water, the polishing step remains crucial for eliminating trace organic and inorganic contaminants. This ensures that ultrapure water of suitable quality is available for sensitive analysis, such as chromatography.
Polishing: From pure (Type 2) to ultrapure (Type 1) water
Since laboratories may have different technologies serving as their tap-to-pure water pretreatment step, we evaluated whether the combined technologies in the polishing step of a Milli-Q® IQ 7 series purification system (Figure 1, right side) could effectively remove pesticides relevant for cannabis testing from contaminated pure water. To conduct this assessment, we injected the pesticide standard solutions into the tank, to reach a concentration of about 1 µg/L of each pesticide. We then analyzed both the spiked tank water and the freshly dispensed ultrapure water after treatment though the EDS-Pak® polisher at the point-of-dispense.
LC-MS/MS and GC-MS/MS analyses revealed that the concentration of each pesticide was below the LOQ in the ultrapure water obtained (Table 1 and Figure 2, right sides). These findings lead us to conclude that the purification technologies in the Milli-Q® IQ 7 series water purification system, under these conditions, successfully produce ultrapure water free of pesticides commonly examined by cannabis testing laboratories.
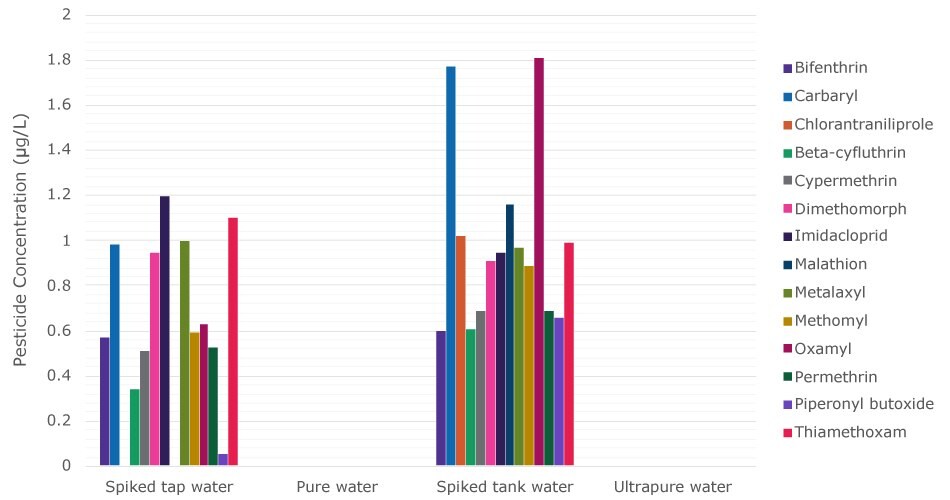
Figure 2.Pesticide concentrations in four water samples after different water purification steps. Specifically, from left to right, spiked tap water, pure water after pretreatment, spiked pure water in the tank, and freshly dispensed ultrapure water after polishing that included an EDS-Pak® polisher at the point-of-dispense. The LOQ value was 0.05 µg/L for each pesticide. It has to be noted that the studied pesticides appear to be impacted by a matrix effect, explaining the variation on the concentration of certain pesticides present in the spiked solutions.6
Conclusion
This study demonstrates that the purification technologies integrated into Milli-Q® IQ 7 series water purification systems combined with the EDS-Pak® polisher at the point of dispense, effectively eliminate pesticides, even under challenging conditions specific to cannabis testing. The ultrapure water produced by these systems is suitable for critical analytical applications in cannabis testing laboratories. Quality control laboratories conducting pesticide analysis on cannabis samples—whether for medicinal or cannabis-derived products—can confidently rely on a benchtop Milli-Q® system equipped with an EDS-Pak® polisher to consistently provide ultrapure water free of detectable pesticide residues.
Finding an Optimal Water Purification Solution
A variety of water purification solutions are available to support scientists conducting pesticide analysis within the cannabis testing sector, ensuring compliance with regulatory standards and the safety of cannabis products for consumers.
Experimental procedure
Analyses were performed by a third-party accredited laboratory (Laboratoire GIRPA, Beaucouzé, France).
Sample preparation & calibration
Pesticides were extracted with 3 successive extractions using 80/20 dichloromethane/ethyl acetate solution. The pH was adjusted to 7 using a 10 M sodium hydroxide solution, and the organic phase was collected. Then, an 85% orthophosphoric acid solution was added to the aqueous phase. Extraction tracers were added to the mixture to quantify the dosage, then 30 g of sodium chloride was added in 500 mL of the solution. All fractions were gathered and placed at -18 °C to remove any trace of water using a filter. The solution was concentrated using a rotavapor (1 mL). A portion of the resulting aliquot was used for GC-MS/MS analysis. Another fraction of the aliquot was added in a solution of 50/50/0.1 v/v/v ultrapure water/methanol/acetic acid (by exchange with ethyl acetate using a flux of nitrogen). This fraction was used for LC-MS/MS. Internal standards were added to correct injection biases. Calibration curves were obtained in the required range.
Detection parameters
The samples were injected using the parameters described in Table 2 (LC-MS/MS) and Table 3 (GC-MS/MS).
Related Products
References
To continue reading please sign in or create an account.
Don't Have An Account?