Visible-Light-Mediated Reactions Under Mild Conditions
Abstract
Visible-light-mediated reactions have been incorporated into various organic synthesis schemes. Recently, owing to the important focus on environmental issues, the development of an environmentally friendly and mild synthetic methodology has become urgently needed. This review summarizes our recent strategies for enabling sustainable organic synthesis through visible-light-mediated reactions conducted under mild conditions, without relying on toxic transition-metal-based catalysts or harsh reaction environments. Relevant applications in the synthesis of valuable small molecules, including bioactive compounds, have also been highlighted.
Section Overview
Introduction to Visible-Light-Driven Organic Synthesis
The use of visible light to effect chemical transformations has been a long-term challenge for chemists. As an example, in 1912, Giacomo Ciamician reported “The Photochemistry of the Future” in Science.1 Afterward, Kellogg provided a basis for modern photoredox catalysis by reporting a reductive amination reaction in 1978.2 Interestingly, however, not many studies were reported until the 2010s. Recently, visible-light-mediated photoredox catalysis has attracted a great deal of attention in organic synthesis.3 In these types of reactions, visible light is converted into chemical energy by engaging in single-electron transfer (SET) with substrates to generate reactive intermediates.
Organic Photocatalysts for Sustainable Transformations
Visible-light-mediated transformations, with their distinct mechanistic pathways, have enabled the development of a broad spectrum of reactions. In recent years, efforts have increasingly focused on green activation strategies, driven by the rising demand for environmentally benign synthetic methodologies. The most common and general approach to greener photoredox catalysis is the use of organic photocatalysts. Various organophotocatalysts, including newly developed catalysts and traditional organic dyes, such as rose bengal, eosin Y, and 4CzIPN (2,4,5,6-tetrakis(9H-carbazol-9-yl)-isophthalonitrile) (Figure 1), have been employed in numerous reactions.3b,4
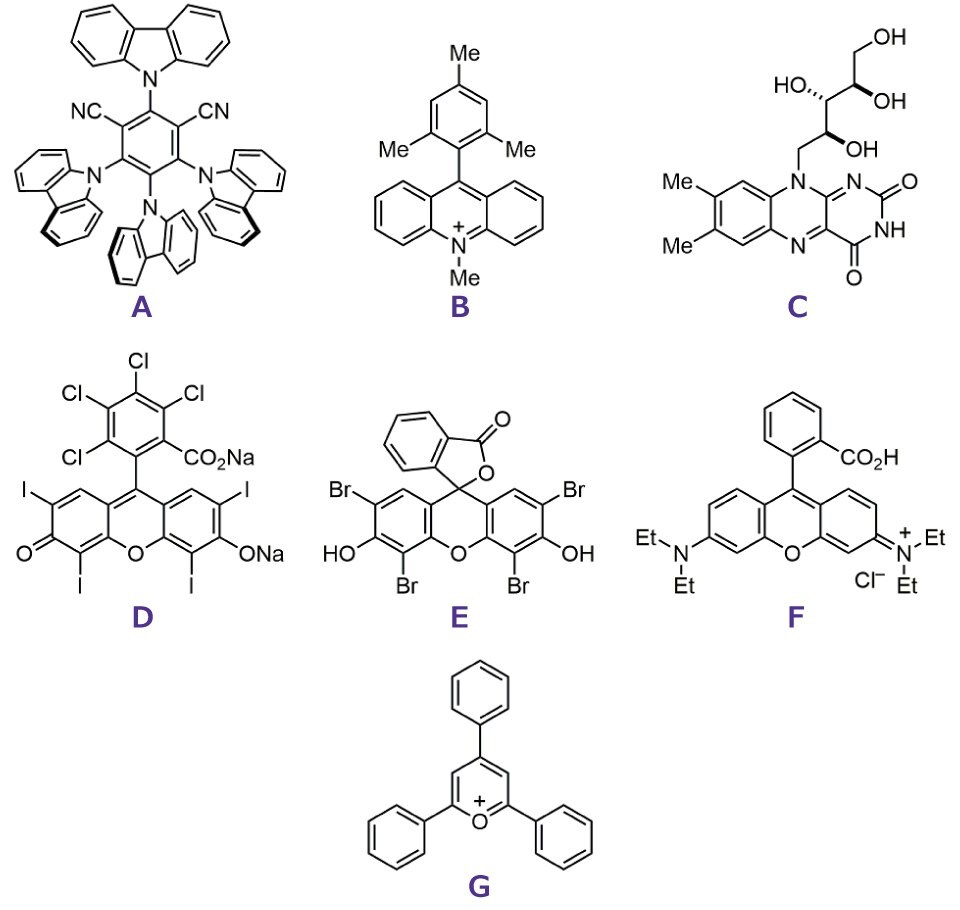
Figure 1.Examples of common organic photocatalysts, (A) 4CzIPN (B) MeS-Acr-Me+ (C) Riboflavin (D) Rose Bengal (E) Eosin Y (F) Rhodamine B (G)TPP+.
Recently, many research groups have focused on the advantages of organic photocatalysts because their structures can be modified by a rational approach to realize unprecedented reactivity or improve the catalytic activity. As an alternative green approach, catalyst-free reactions have been developed. These latter types of reaction modes include the use of photoactive starting compounds or in situ excitation of the substrates under visible-light irradiation. The substrates thus activated form electron donor−acceptor (EDA) complexes, obviating the need for photoredox catalysts.5 This strategy relies on associating electron-donor with electron-acceptor substrates, such as Lewis bases and acids, respectively. The EDA complex can absorb visible light, undergoing an excitation process, which triggers a single-electron transfer (SET) that can generate radical intermediates.
In addition to the above-mentioned approaches, green synthetic methods using natural substances such as molecular oxygen have also been studied. Our group has focused on the development of novel, visible-light-mediated reactions under mild reaction conditions. Herein, we summarize our approaches for realizing the green mode of visible-light-mediated reactions (Figure 2).
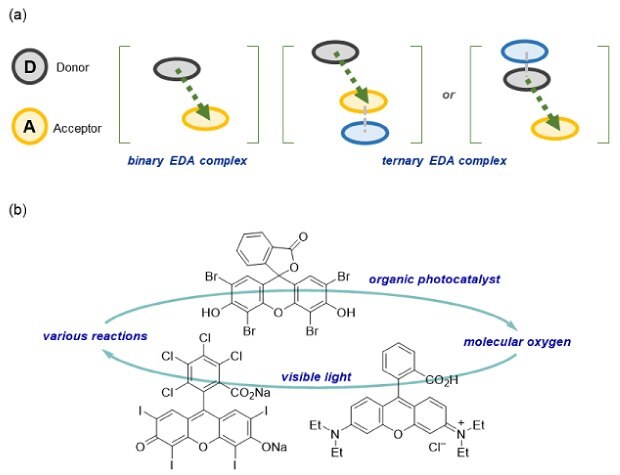
Figure 2. Our green approaches for the development of mild, visible-light-mediated reactions, (a) catalyst free reactions (b) organo-photocatalysis.
Conclusions and Future Directions in Green Photochemistry
Developing green synthetic methods is a key objective in the field of visible-light-mediated reactions. In this short review, we introduced our approaches for carrying out valuable organic transformations under mild reaction conditions such as visible-light-mediated aerobic oxidations using molecular oxygen. Additionally, we expanded the scope of singlet-oxygen-mediated reactions by developing oxidative C–S bond cleavage reactions. Furthermore, we advanced catalyst-free methods via electron donor–acceptor (EDA) complex-based strategies, leveraging binary and ternary complexes as reactive intermediates. While various mechanistic studies have provided insight into these processes, further investigation is needed to directly characterize EDA complexes.
Herein, we also highlighted applications of these approaches to the synthesis of valuable small molecules. Ongoing research in our laboratory aims to develop novel green syntheses with visible light photochemistry. Future directions include the structural modification of organic photocatalysts and the development of novel visible-light-active starting compounds to expand the range of available reactions in this field.
Related Products
For the full article and complete reference list, see Aldrichimica Acta, Volume 56, No. 1, 2023, p. 3.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?