Silver Nanomaterials: Properties & Applications
Introduction
Silver nanomaterials possess unique physical, chemical, and optical properties that are being leveraged for a wide array of biological applications. A resurgence of interest in silver as a broad-spectrum antimicrobial agent has led to the development of numerous products that incorporate silver nanoparticles to prevent bacterial growth on surfaces and in textiles. Additionally, researchers continue to explore silver nanoparticles for biosensing and optics applications such as ultrabright reporter molecules, highly efficient thermal absorbers, and nanoscale “antennas”.
Morphology and Surface Functionalization
Because the size, shape, and surface functionalization of silver nanoparticles tune their properties, the synthetic conditions are critical to control. The synthesis conditions during silver nanomaterial fabrication can be precisely adjusted to produce colloidal silver nanoparticles with various morphologies, including monodisperse nanospheres, triangular prisms, nanoplates, cubes, wires, and nanorods. The surface chemistry, morphology, and optical properties of these nanoparticles must be carefully selected to achieve the desired functionality in target environments.
In aqueous media, nanoparticles are often electrostatically stabilized through the addition of charged species at their surfaces. The surface charge can be controlled by coating the particles with citrate ions, which provide a strong negative charge. Alternatively, replacing citrate with branched polyethylenimine (BPEI) creates an amine-dense surface with a highly positive charge. Other capping agents, such as polyethylene glycol (PEG) and lipoic acid, can enhance stability and provide functional groups for bioconjugation.
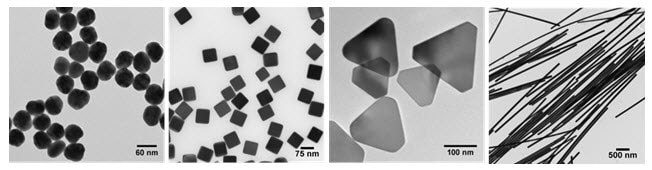
Figure 1.Transmission electron micrographs demonstrating the diversity of size and morphology possible by control over the reaction chemistry and kinetics during the solution-phase synthesis of silver nanomaterials: (left to right) uniform 50 nm diameter spheres (796131), 75 nm cubes (NCXSCPH75), 120 nm triangular nanoplates, and silver nanowires (807508).
Optical Properties
Silver nanoparticles exhibit strong interaction with light due to collective oscillation of conduction electrons at specific wavelengths, known as surface plasmon resonance (SPR). This phenomenon results in significantly higher absorption and scattering intensities compared to identically sized non-plasmonic nanoparticles.
The extinction spectra of different sizes of silver nanospheres and nanoplates, and the appearance of dilute dispersions of the nanoparticles are shown in Figure 2. Smaller nanospheres primarily absorb light and have plasmon resonance peaks near 400 nm, while larger spheres exhibit increased scattering and have peaks that broaden and shift towards longer wavelengths. Silver nanoplates, due to their anisotropic shape, have extremely large absorbing and scattering cross-sections across the visible and near-IR regions of the spectrum. By precisely controlling the plate diameter and thickness, the plasmon resonance can be tuned to peak at specific wavelengths (Figure 2).
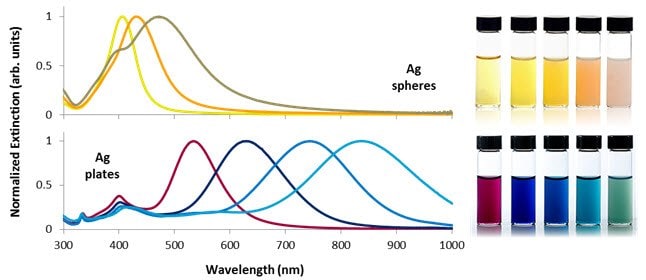
Figure 2.Selected optical extinction spectra and solution appearance of silver nanospheres between 10 and 100 nm in diameter (top) and silver nanoplates between 50 and 150 nm in diameter (bottom). Control over nanoparticle shape and size allows the plasmon resonance to be tuned across the visible and near-infrared portions of the spectrum.
Antimicrobial Properties
The antimicrobial effects of silver have historical roots, tracing back to ancient civilizations. Silver ions interact with thiol groups of essential bacterial enzymes and proteins, disrupting cellular respiration and leading to cell death. The specific toxicity to bacteria, while maintaining low toxicity for humans, has facilitated the integration of silver nanoparticles into various products, including wound dressings and antifouling coatings.
A central mechanism of antimicrobial activity is the high surface area of silver nanoparticles, which serves as a source for silver ions. In aqueous environments, these particles oxidize in the presence of oxygen and protons, releasing Ag+ ions. The release rates depend on several factors, including nanoparticle size, shape, capping agent, aggregation state, and environmental conditions. Smaller or anisotropic particles typically exhibit faster ion release rates due to their high surface energy.
Silver Nanomaterials in Tagging and Targeting for Bioimaging
Silver nanoparticles are utilized in tagging and imaging applications due to their efficient light absorption and scattering capabilities. The high scattering cross-section enables individual silver nanoparticles to be visualized through techniques like dark field microscopy or hyperspectral imaging (Figure 3). Coupling biomolecules such as antibodies or peptides to the surface of silver nanoparticles allows for targeted delivery to specific cells or cellular components. This attachment can be achieved via physisorption or covalent coupling, enhancing specificity and reducing non-specific background.
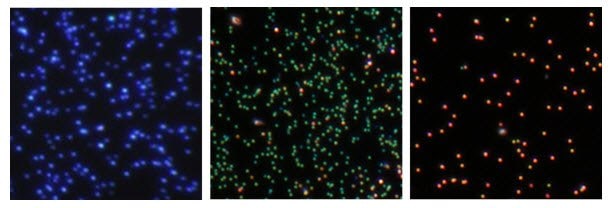
Figure 3.Dark field microscopy images of (left to right) 60 nm diameter silver nanospheres, 75 nm diameter silver nanocubes, and 100 nm diameter silver nanocubes, illustrating the ability to tune the scattering color of silver nanoparticle labels based on size and shape.
Enhanced electromagnetic fields near the surface of silver nanoparticles also benefit spectroscopic techniques like Raman spectroscopy. Surface Enhanced Raman Scattering (SERS) significantly amplifies Raman signals from molecules near the nanoparticles, allowing for the detection of single molecules. Similarly, placing a fluorophore close to the nanoparticle surface can lead to Surface Enhanced Fluorescence (SEF), increasing emission intensity by orders of magnitude.
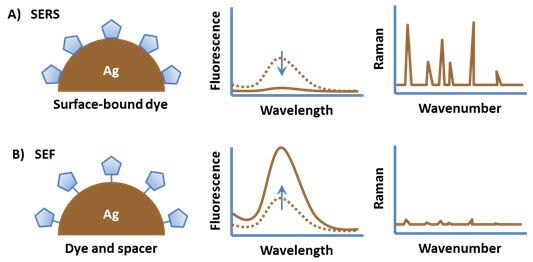
Figure 4. (A)An organic fluorophore attached directly to a metal substrate typically has quenched fluorescence but a strong surface enhanced Raman spectrum. (B) Spacing the fluorophore off of the metal surface results in surface enhanced fluorescence.
Nanomedicine and Nanotoxicology
The applications of silver nanoparticles in both in vitro and in vivo contexts are rapidly expanding. They are employed not only as labeling agents and nanotags but also as thermal sources for hyperthermia and for thermally modulated drug release. Silver nanoparticles can be incorporated into core/shell constructs, where an amorphous silica shell is uniformly grown around silver nanoparticle cores, allowing for functional group conjugation and integration of drug molecules within the shell for targeted delivery.
Understanding the interactions between silver nanoparticles and biological systems is crucial for future biomedical applications. For in vivo applications, challenges include designing particles with long circulation times and low toxicity. The biological fate and transport of nanoparticles depend on their primary characteristics (core chemistry, size, shape, etc.) and their interactions with biological systems (e.g., protein corona, dissolution rate).
Conclusions and Future Outlook
The unique optical properties and broad-based antimicrobial properties of silver nanoparticles have led to a rapid rise in the incorporation of silver nanoparticles in biological applications. The high level of control that is available for controlling size, shape, and surface of silver nanoparticles provides a powerful library for not only generating functional materials for biological applications but also for understanding the fundamental mechanisms of transport and interaction of nanoparticles in biological systems. This understanding, coupled with the construction of more complex multifunctional silver nanocomposites, will enable the next generation of silver nanoparticle-based probes, devices, and therapeutics.
Related Products
References
To continue reading please sign in or create an account.
Don't Have An Account?