Progress on Nonviral Delivery for CRISPR/Cas9
Introduction
The CRISPR/Cas9 system has recently emerged as a robust and versatile genome-editing platform for building disease models and correcting diseased genes. CRISPR stands for and refers to clustered regularly interspaced short palindromic repeat found by Nakata et al. in /CN/en1987/CN/en1 as repeat sequences interspaced by nucleotide spacers in the Escherichia coli genome. This genomeediting system contains two main components: a nuclease protein Cas9 that binds to DNA and initiates double-strand breaks (DSBs), and a very short single guide RNA (sgRNA) that directs the Cas9 nuclease to the targeted genomic locus. Since the CRISPR/Cas9 system can only function within the cellular environment, this genome-editing system requires a delivery mechanism to targeted cells. To date, both physical approaches and viral vectors have been extensively studied for CRISPR/ Cas9 delivery, showing significant progress in improving delivery accuracy and transfection efficiency. However, these methods have intrinsic limitations, including insertional mutagenesis, carcinogenesis, and immunogenicity, that dramatically restrict their clinical applications. Therefore, new systems for CRISPR/ Cas9 delivery are needed to allow the safe and effective clinical development and applications of CRISPR/Cas9 systems.
To achieve effective in vivo delivery of the CRISPR/Cas9 system, it is necessary to understand and overcome the three major obstacles to the use of CRISPR/Cas9 from in vitro loading to in vivo delivery. First, the size of the CRISPR/Cas9 system is more substantial than traditional gene editing tools. In detail, the total plasmid size is larger than 7 kb, and commonly used Cas9 proteins are about /CN/en160 kDa, significantly increasing the difficulty of in vivo delivery. Thus, it is essential to condense the CRISPR/ Cas9 system into relatively small particles through the use of suitable carriers. Second, the naked CRISPR/Cas9 genomeediting cargos are exogenous biomacromolecules, that are vulnerable to recognition and rapid clearance by the host immune system. As a result, their half-life is too short to function in vivo. To prolong the half-life, the delivery platform must protect the genome-editing cargo from degradation from the physiological environment. Third, the gene editing process takes place in the cytoplasm or nucleus, but the genome-editing cargos cannot readily traverse the hydrophobic lipid cell membrane by themselves. Therefore, a suitable delivery vector is required to allow the CRISPR/Cas9 system to penetrate the host cells.
In previous studies, nonviral nanoparticles like liposomes, polymeric nanoparticles, and inorganic nanoparticles have demonstrated many advantages such as lower immunogenicity, high packaging capability, and design flexibility, showing great potential to satisfy the requirements for CRISPR/Cas9 delivery. Numerous researches have recently focused on nonviral approaches as promising alternatives in developing an effective CRISPR/Cas9 delivery system. In this review, we provide an overview of the latest nonviral approaches adopted for CRISPR/ Cas9 delivery in order to offer a reference for future research.
Lipid-Based Platforms
Lipid-based carriers are a primary type of nonviral gene carrier. Lipofectamine, a commercially available liposome, has been widely used in vitro for the transfection of mRNA due to its high transfection efficiency. However, the high toxicity and inflammatory side effects have limited its further application. Researchers are engaged in modifying conventional liposomes with biodegradable chemical bonds or targeting ligands to provide more functionality. Wang et.al2 reported a combinatorial library of cationic bioreducible lipids to deliver Cas9/sgRNA. These lipid-based vectors can effectively facilitate the escape of Cas9/sgRNA from endosomal and direct protein to its intracellular targets under a reductive intracellular environment with more than 70% delivery efficiency (Figure 1A). Finn et al.3 designed a biodegradable lipid-based formulation via labile ester linkages. With the introduction of apolipoprotein E (ApoE) as the target moiety, this biodegradable lipid-based CRISPR/Cas9 vectors exhibit significantly enhanced uptake by hepatocytes cells and demonstrate more than 97% gene knockdown efficiency.
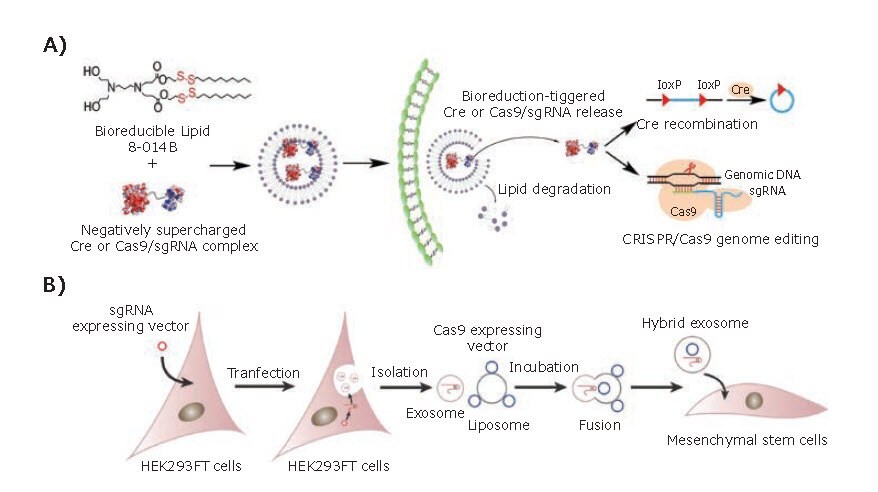
Figure 1.Lipid-based platforms. A) Molecular design of bioreducible lipid and its delivery mechanism. Reproduced with permission from reference 2, copyright 2016 National Academy of Science. B) Illustration of the procedure of how the hybrid exosomes deliver the CRISPR/Cas9 interference system. Reprinted with permission from reference 3, copyright 2018 Wiley VCH.
Exosomes are small extracellular vesicles secreted by mammalian cells, which can be used as attractive nanocarriers owing to their stability, biocompatibility, low immunogenicity, and low toxicity. However, it is challenging to encapsulate large nucleic acids or proteins into exosomes due to their small size (30–100 nm). To utilize the advantages of exosomes in CRISPR/Cas9 delivery, Lin et al.4 developed a hybrid exosome by incubating them together with liposome. Different from the original exosomes, the hybrid encapsulated CRISPR/Cas9 plasmid DNA and efficiently expressed the encapsulated genes in mesenchymal stem cells (MSCs) (Figure 1B).
Polymer-Based Platforms
Polymer-based nanoparticles are widely used for delivering various types of nucleic acids, including plasmid DNA, RNA, and oligonucleotide, due to their excellent encapsulation capability, specific targeting of tissues or organs, and remarkable properties in stabilizing nucleic acids against serum-induced aggregation. Based on previous research, polymer-based platforms for CRISPR/Cas9 delivery has attracted much attention.
Polyethyleneimine (PEI) is the most common polymeric gene vector. The rich amine groups of PEI endow them with high charge density, which allows efficient DNA condensation and endo/lysosomal escaping. Nowadays, PEI 25K is even considered the gold standard for DNA/RNA transfection. However, excessively strong positive charge and significant cytotoxicity limit further in vivo applications. Fortunately, recent research has shown that this cytotoxicity can be effectively reduced using linear and lower molecular weight PEI or by chemical modification with moieties such as PEG. Wei and colleagues5 reported a multifunctional nucleus-targeting “core-shell” artificial virus (RRPHC) for delivery of the CRISPR/Cas9 system. The RRPHC artificial virus has a core-shell structure, in which the core composition contains fluorinated low molecular weight PEI, and the shell formation occurs using a versatile multifunctional PEG layer (RGD-R8-PEG-HA, RRPH, Figure 2A). This artificial virus effectively directs the endosomal escape and nucleus entry of the CRISPR/Cas9 system without further addition of any nuclear-localization signal, exhibiting very high transfection efficiency to SKOV3 cells (more than 90%).
Similarly, Liu et al.6 developed a multistage delivery nanoparticle (MDNP), comprised of phenylboronic acid (PBA) modified low molecular weight PEI and 2,3-dimethylmaleic anhydride (DMMA)-modified mPEG113-b-PLys100 (Figure 2B). The corresponding surface properties at different delivery stages ensure this multistage delivery nanoparticle achieves efficient delivery of the CRISPR/dCas9-miR-524 system. In other research, Zhang and colleagues7 synthesized polyethyleneimine-β-cyclodextrin (PC) as a carrier for delivering Cas9/sgRNA pDNA in vitro. Owing to the similar structure as high molecular weight PEI, PC serves as an efficient delivery vector with high efficiency (Figure 2C). The above three studies are all based on low molecular weight PEI with functional chemical modification. Compared with high molecular weight PEI, they show similar transfection efficiency but lower cytotoxicity, demonstrating significant potential as a safer alternative to PEI 25K.
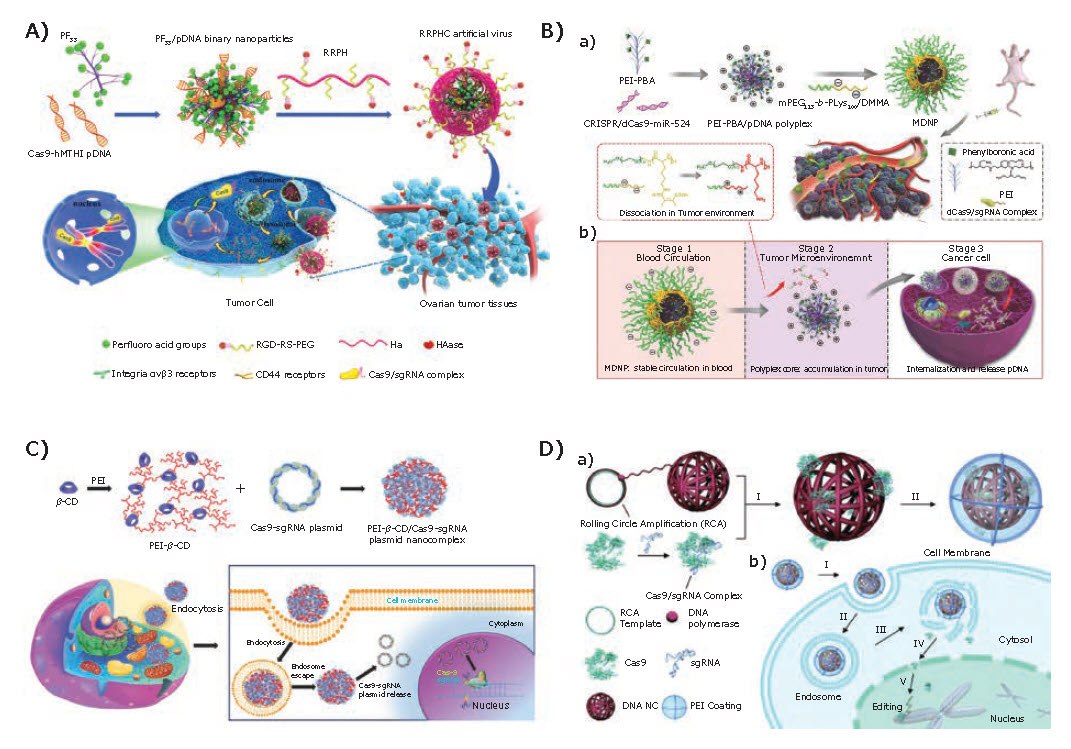
Figure 2.Polymer-based platforms. A) Design of the multifunctional“core-shell” artificial virus (RRPHC) for delivery of the CRISPR/Cas9 system. Reproduced with permission from reference 5, copyright 2017 American Chemical Society. B) Preparation of MDNP and the delivery of CRISPR/dCas9 system after intravenous injection. Reproduced with permission from reference 6, copyright 2018 American Chemical Society. C) Schematic illustration of PC-mediated Cas9/sgRNA plasmid delivery for genome editing. Reproduced with permission from reference 7, copyright 2015 Wiley VCH. D) Design of DNA nanoclew for CRISPR/Cas9 delivery system. Reproduced with permission from reference 8, copyright 2015 Wiley VCH.
In the last decade, new nanostructures developed from DNA, including DNA tetrahedrons, DNA origami structures, DNA nanorobots, and DNA nanoclews, have shown great potential in CRISPR/Cas9 system delivery due to their uniform size, biodegradability, and spatial addressability. In 2015, Sun et al.8 developed a synthetic DNA nanoclew (NC)-based carrier for the delivery of Cas9/sgRNA complexes in vitro and in vivo (Figure 2D). With the assistance of PEI and nuclear-localization-signal peptides, DNA NCs have exhibited effective endosome escape and nucleus targeting capability, achieving much higher editing efficacy (36%) compared to the cell-penetrating peptide (CPP) based vector (9.7%).
Inorganic Nanoparticles
In recent years, rigid nanocarriers such as carbon, gold, and other nanoscale inorganic materials have performed well in many gene delivery applications due to their high surface to volume ratio, size control, and colloidal stability in a physiological environment. For example, a nanocarrier composed of cationic arginine gold nanoparticles (ArgNPs) and engineered Cas9 proteins has been reported (Figure 3A).9 This vector could efficiently deliver protein and nucleic acid to the cytoplasm and subsequently transport it to the nucleus, ultimately achieving up to 90 % delivery efficiency. In 2018, Alsaiari et al.10 reported the first example of applying nanoscale metal-organic frameworks (MOFs) for CRISPR/Cas9 RNP complex delivery (Figure 3B). Nanoscale zeolitic imidazolate framework-8 (ZIF-8) successfully co-encapsulated both Cas9 protein and the negatively charged sgRNA with a high loading efficiency of 17% due to its tunable pore size. With the fast-endosomal escape and enhanced nucleus delivery, the CRISPR/Cas9 system encapsulated by ZIF-8 knocked down the gene expression of green fluorescent protein by 37% over 4 days. In another paper, Zhou et al.11 reported the use of biodegradable two-dimensional black phosphorus nanosheets(BPs) for CRISPR/Cas9 delivery (Figure 3C). Cas9 RNP was loaded onto the BPs via electrostatic interaction with a remarkable loading capacity of 98.7%. The constructed delivery platform could enter the cells via both direct membrane penetration and endocytosis pathway, followed by effective endosomal escape upon biodegradation of the ultra-thin BPs. The resulting good biocompatibility and biodegradability makes it a promising topic for further research.
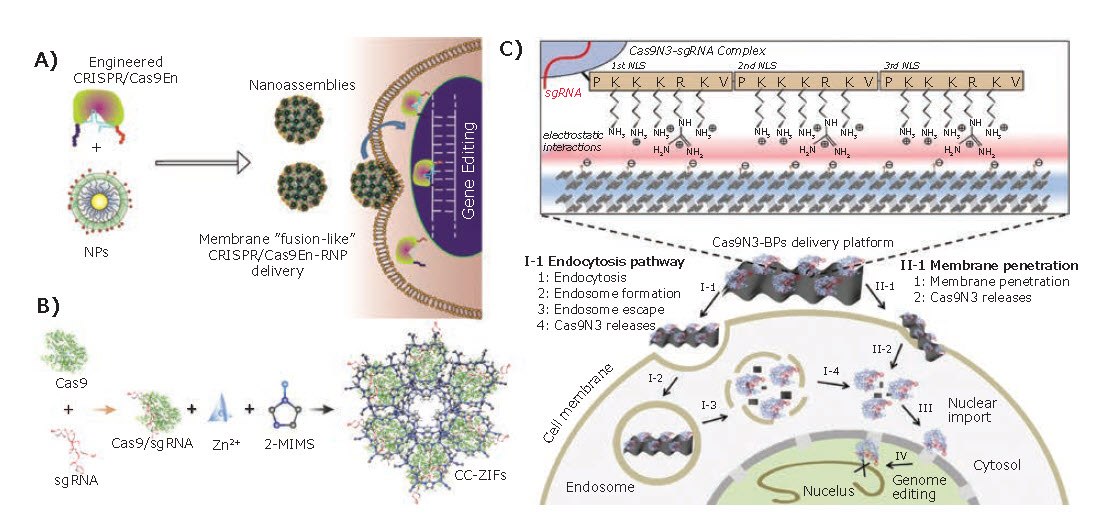
Figure 3.Inorganic Nanoparticles. A) Rational designing of arginine nanoparticles (ArgNPs) for effectively intracellular delivery of the Cas9 protein or Cas9-RNP. Reproduced with permission from reference 9, copyright 2017 American Chemical Society. B) The formation of nanoscale ZIF-8 coencapsulating Cas9 protein and sgRNA (CC-ZIFs). Reproduced with permission from reference 10, copyright 2017 American Chemical Society. C) Design of the Cas9N3-BPs delivery platform and its intracellular delivery pathways. Reproduced with permission from reference 11, copyright 2018 Wiley VCH.
Hybrid Materials-Based Platforms
Like all other delivery applications, no one single carrier can solve all the problems and enable efficient CRISPR/Cas9 delivery. Combining the advantages of different materials into one hybrid multifunctional vector provides the ability to meet various needs simultaneously, ultimately achieving much more efficient delivery.
Polymeric nanoparticles coated with a SiO2 shell have been suggested as promising hybrid carriers for safe and efficient delivery of biologically active compounds. Timin et al.12 constructed a nanoparticle-based system by depositing poly-Larginine hydrochloride and dextran sulfate on CaCO3 particles using the layer-by-layer (LbL) self-assembly method. After removing the CaCO3 core and coating with a SiO2 shell, these carriers achieved more efficient transfection than a commercially available liposome-based transfection reagent due to its high loading capacity and biocompatibility. Liang et al.13 developed a PEG-PEI-Cholesterol (PPC) lipopolymer for delivering CRISPR/Cas9 plasmids (encoding VEGFA gRNA). These aptamer-functionalized PPC lipopolymers successfully decreased the VEGFA expression or secretion and demonstrated significant inhibition in orthotopic osteosarcoma malignancy and lung metastasis. In another report, Chen et al.14 developed liposome-templated hydrogel nanoparticles (LHNPs) for targeted CRISPR/Cas9 delivery. The PEI-based hydrogel has a core-shell structure, in which the core is a cationic polyplex constructed by crosslinking cyclodextrin (CD)-engrafted PEI (PEI-CD) with adamantine (AD)-engrafted PEI (PEI-AD), whereas the shell is DOTAP lipids (Figure 4A). This unique core-shell structure is capable of delivering Cas9 protein and plasmid DNA simultaneously, exhibiting high delivery efficiency and low toxicity.
Graphene oxide (GO) has attracted significant attention in biological applications, especially as a carrier for the delivery of small molecular drugs and nucleic acids into cells. Compared with other nanocarriers, the planar structure of GO provides a higher specific surface area, thereby effectively enhancing the payload capacity. In 2018, Yue et al.15 developed the first delivery platform based on PEG and PEI dual-functionalized GO, which is capable of loading Cas9/sgRNA by physical adsorption and π-stacking interactions. This nanoplatform successfully achieved intracellular delivery of Cas9/sgRNA via endocytosis, exhibiting 39% gene disruption with excellent biocompatibility (Figure 4B).
Also, researches have shown that hybrid multifunctional vectors are more intelligent, which can overcome both intracellular and extracellular barriers at the same time. For example, Harashima et al.16 developed a multifunctional envelope-type nanodevice (MEND) composed of a mitochondria targeting liposome called MITOPorter and enzymatically cleavable PEG (Figure 4C). Such intelligent nanocarriers have the potential to avoid host immune system recognition during blood circulation and realize effective cell internalization after accumulating in tumor tissues, finally achieving high delivery efficiency. Wang et al.17 created a photothermal-activatable lipid/gold nanoparticle (AuNP) platform for the delivery of Cas9-gPlk-1 plasmids (Figure 4D). The multifunctional vehicle enters tumor cells and releases the Cas9-gPlk-1 plasmids into the cytosol by laser-triggered thermo-effects of the AuNPs. Then, by TAT guidance, the Cas9-gPlk-1 plasmids enter the nuclei to finish the gene editing. The synergistic effects of AuNPs, TAT peptide, and the outer lipid shell guarantee high efficiency and targeted gene editing.
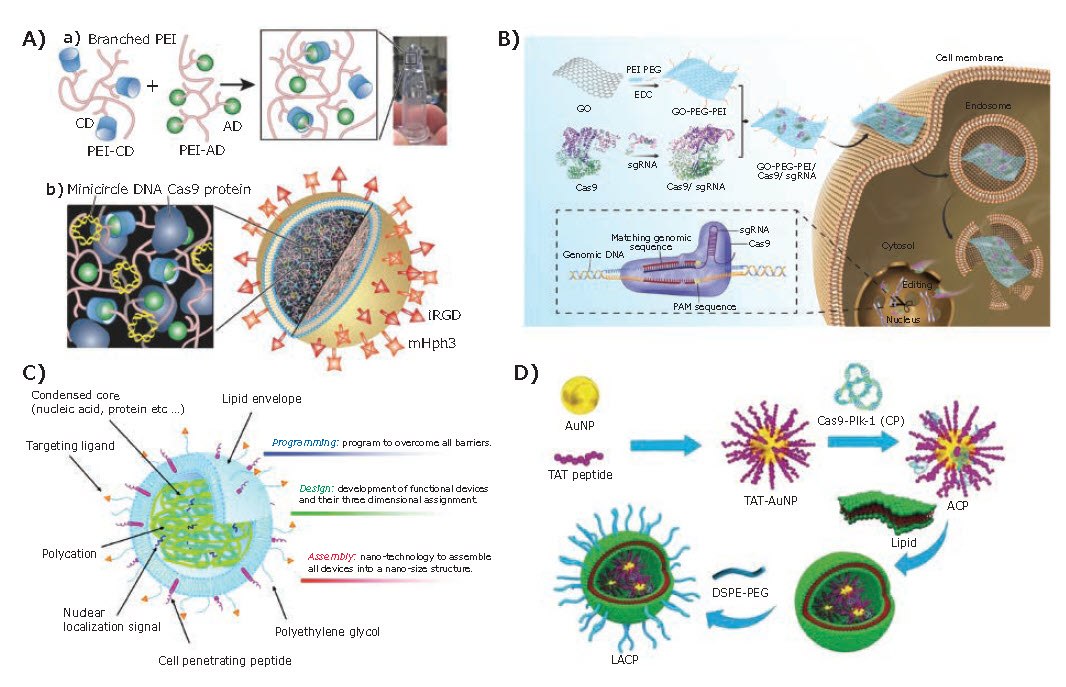
Figure 4.Delivery platforms based on hybrid materials. A) Formation of liposome-templated hydrogel nanoparticles (LHNPs) by DOTAP liposomes and PEI hydrogel. Reproduced with permission from reference 14, copyright 2017 Wiley VCH. B) The schematic process of preparation and intracellular delivery of GO-PEG-PEI based Cas9/sgRNA delivery. Reproduced with permission from reference 15, copyright 2018 Royal Society of Chemistry. C) Design of the multifunctional envelope-type nanodevice (MEND) based on “Programmed Packaging”. Reproduced with permission from reference 16, copyright 2012 American Chemical Society. D) Preparation processes for lipid-encapsulated TAT peptide-modified AuNPs. Reproduced with permission from reference 17, copyright 2017 Wiley VCH.
Conclusion and Perspectives
The CRISPR/Cas9 system offers significant advantages over other genome-editing technologies such as simplicity and versatility. However, their clinical application mainly relies on the effective delivery of genome-editing cargo to the target cells. Numerous innovative nanoparticle delivery systems like polymer-based, lipid-based, and rigid inorganic nanoparticles have been designed for the CRISPR/Cas9 system. To meet the various needs of the different delivery stages in vivo, hybrid multifunctional delivery platforms are also in development. All these make the clinical application of nonviral vectors for CRISPR/Cas9 delivery very promising in the near future.
To date, the design of most delivery platforms are for pDNA or mRNA. Compared with the above two delivery modes, direct delivery of Cas9 protein/sgRNA shows the advantages of rapid action, high efficiency, and lower off-target effect. However, packaging RNP into a small particle while maintaining its biological activity in the blood is still a significant challenge to overcome. Since there are few alternative protein carriers for delivery, this is a fertile research direction that is greatly needed to advance CRISPR/Cas9 technology.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?