Solid Formulation Development Using Melt-based 3D Printing Technologies
Three-dimensional (3D) printing is a powerful technology that has wide-ranging applications, including many in the pharmaceutical industry. 3D printing offers the potential to produce medications tailored to the needs of patients and dosage forms in various shapes, sizes, and textures with different release profiles that can be difficult to produce using conventional techniques.1
For the production of pharmaceutical dosage forms, 3D printing can be applied to powder-based, liquid-based, and extrusion-based systems. For powder-based systems, drop-on-powder (DOP) and selective laser sintering (SLS) technologies can be used while for liquid-based systems, drop-on-drop deposition (DOD) and stereolithography (SLA) can be applied. Extrusion-based systems include for example fused-deposition modeling (FDM) and melt-drop deposition (MED®).
This technical article shows how 3D printing can overcome challenges during formulation development, with a focus on enhancement of bioavailability of active pharmaceutical ingredients (APIs) in solid dispersions.
Webinar: Latest Advancements of Melt-based 3D Printing Technologies for Oral Drug Delivery
Overview of Hot Melt Extrusion and the 3D Printing Process
The 3D printing process for pharmaceuticals takes place in two steps:
- Choose a polymer that is compatible with the API and that is suitable for printing
- Print the API, e.g. using melt drop deposition. For some approaches, the manufacturing of an intermediate, such as strands manufactured using HME, is necessary.
Choosing an HME Polymer for 3D Printing
In choosing a polymer for HME for 3D printing, it’s important to consider how the shear rate impacts viscosity. Ideally, the viscosity should decrease with an increasing shear rate (Figure 1). This is essential when it comes to processing material through very small nozzles to enable an efficient downstream process.
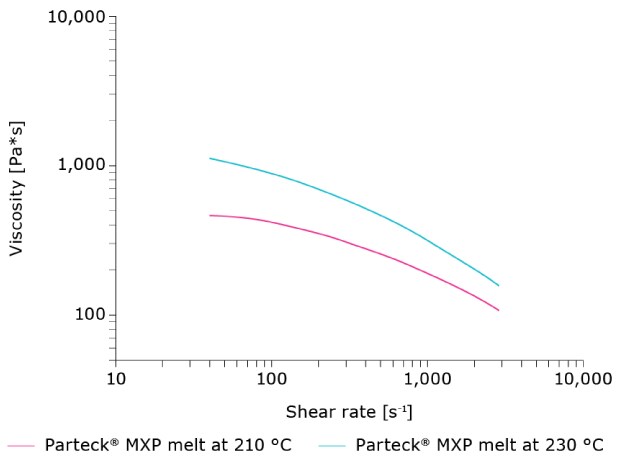
Figure 1.Characteristics of Parteck® MXP polyvinyl alcohol make it well-suited for HME.
Melt Drop Deposition
In the melt drop deposition process for solid dosage forms, which is sometimes also referred to as melt extrusion deposition, the polymer is melted in a heated plasticizer barrel in which a screw rotates and transports the material to the nozzle tip. When the polymer melt reaches the polymer reservoir, pressure is applied via translational movement of the screw, and droplets are discharged via a piezo actuator which can operate at a very high frequency of up to 250 hertz (Figure 2).
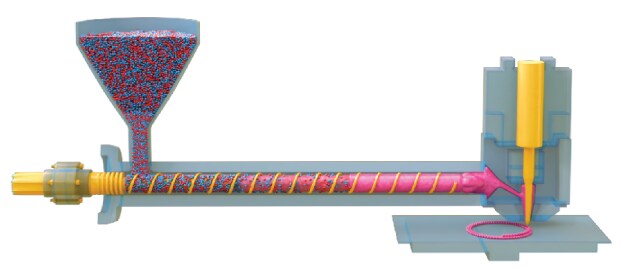
Figure 2.The principle of advanced melt drop extrusion.
SEM images of the top and side surfaces of 3D printed tablets and strands show high homogeneity of this deposition process. A detailed view of the strands shows the individual drops that were deposited (Figure 3).
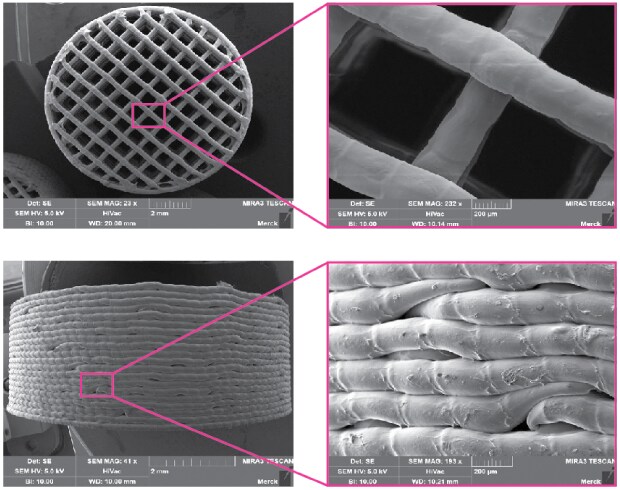
Figure 3.SEM images of tablets and strands produced using melt drop deposition.
The infill volume can be varied to individually adjust the porosity of the tablets (Figure 4). This allows us to modify and match the targeted release kinetics.
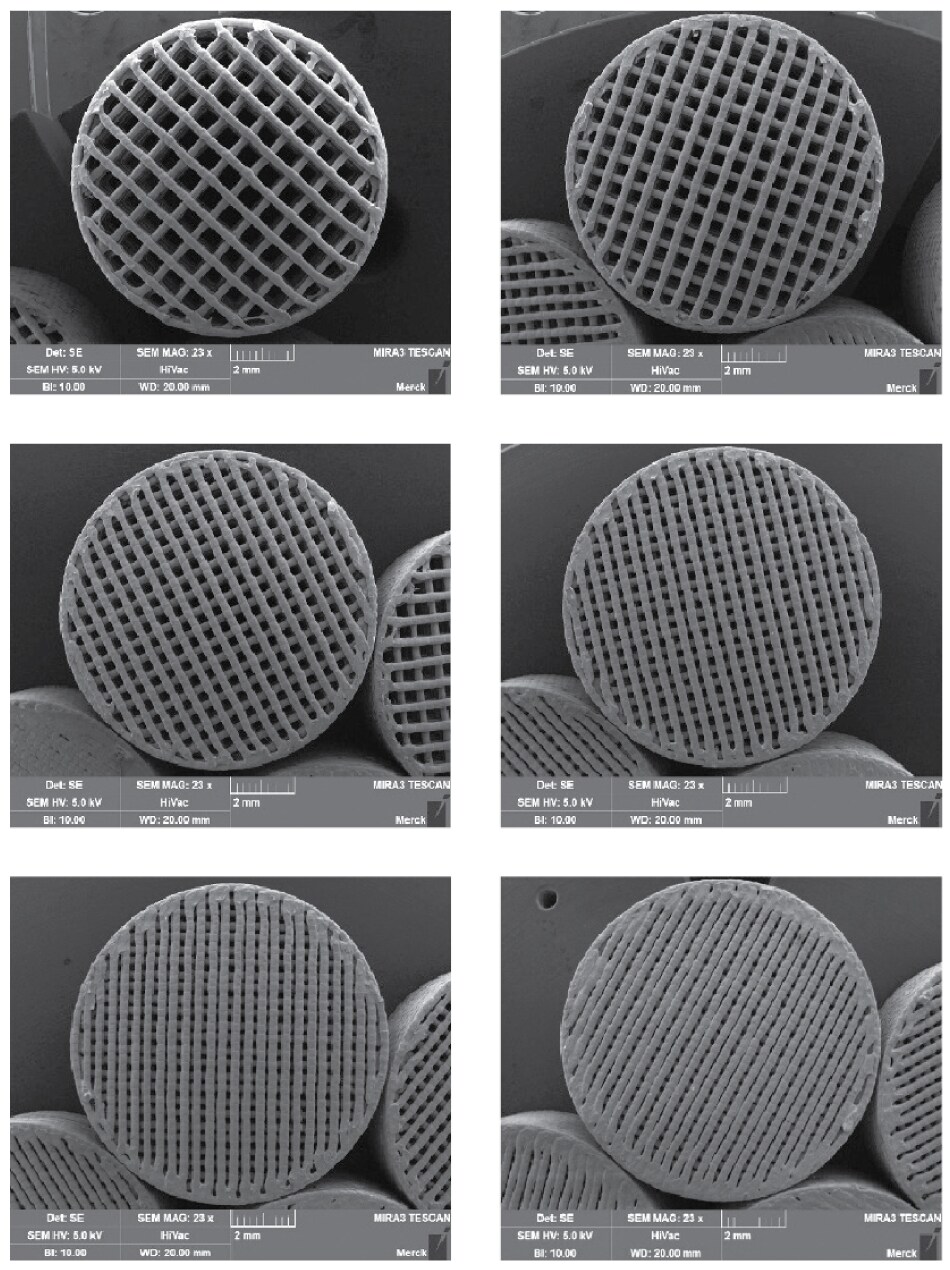
Figure 4.Variation of infill can be used to adjust tablet porosity and surface area.
This system was highly reproducible for tablet production. Figure 5 shows the mass distribution for the Parteck® MXP excipient placebo tablets and those containing 10% caffeine. A comparison of the mass versus the inflow volume showed relatively lower homogeneity for the formulation with caffeine, but still within the required limits.
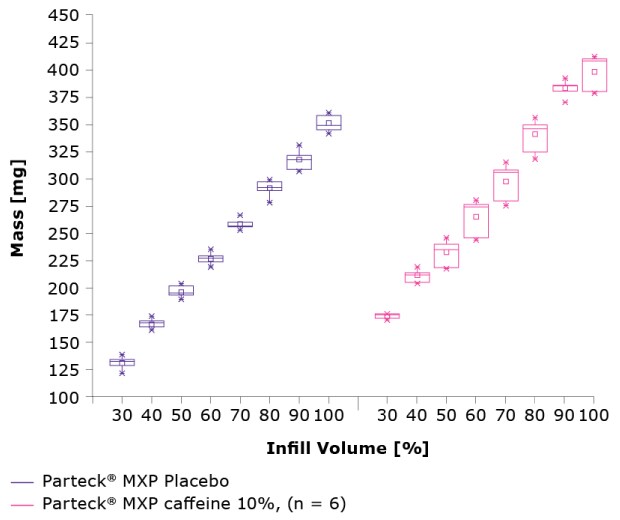
Figure 5.Comparison of infill volume and mass for Parteck® MXP placebo vs. Parteck® MXP with 10% caffeine.
The mechanical stability of the tablets was also a key consideration. Diametral compression was assessed with a texture analyzer and showed that 3D printed tablets based on Parteck® MXP excipient provided high mechanical strength even at low infill volumes (Figure 6). The high mechanical strength also translates into low friability values over the entire process range.
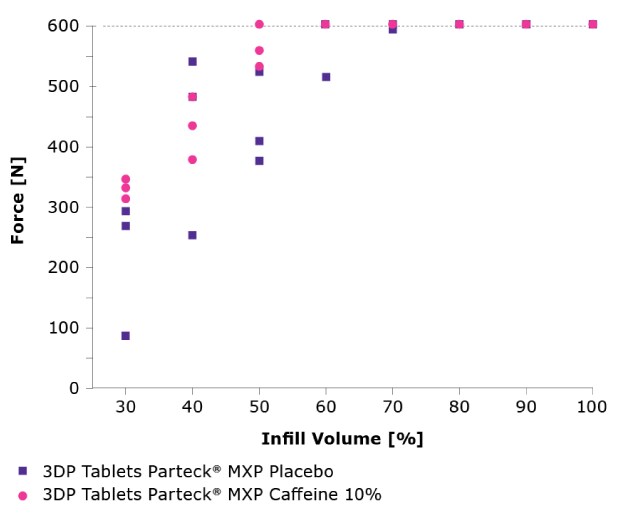
Figure 6.Diametrical compression of placebo tablets (blue square) and those containing 10% caffeine (pink circle); at 600 N the maximum force of the system is already exceeded.
3D Printed Tablets Enable Various Drug Release Profiles
A final consideration was whether melt drop deposition could be used to modify drug release. Figure 7 shows the release profiles of the caffeine-loaded tablets at different infill percentages compared to pure caffeine drug substance, a soluble compound that allows the effect of the polymer on the release rates to be determined. The results indicated that it was possible to modify drug release rates via the infill volume. Low standard deviations indicate a high reproducibility of the results which is an important step for further industrial application of this technology.
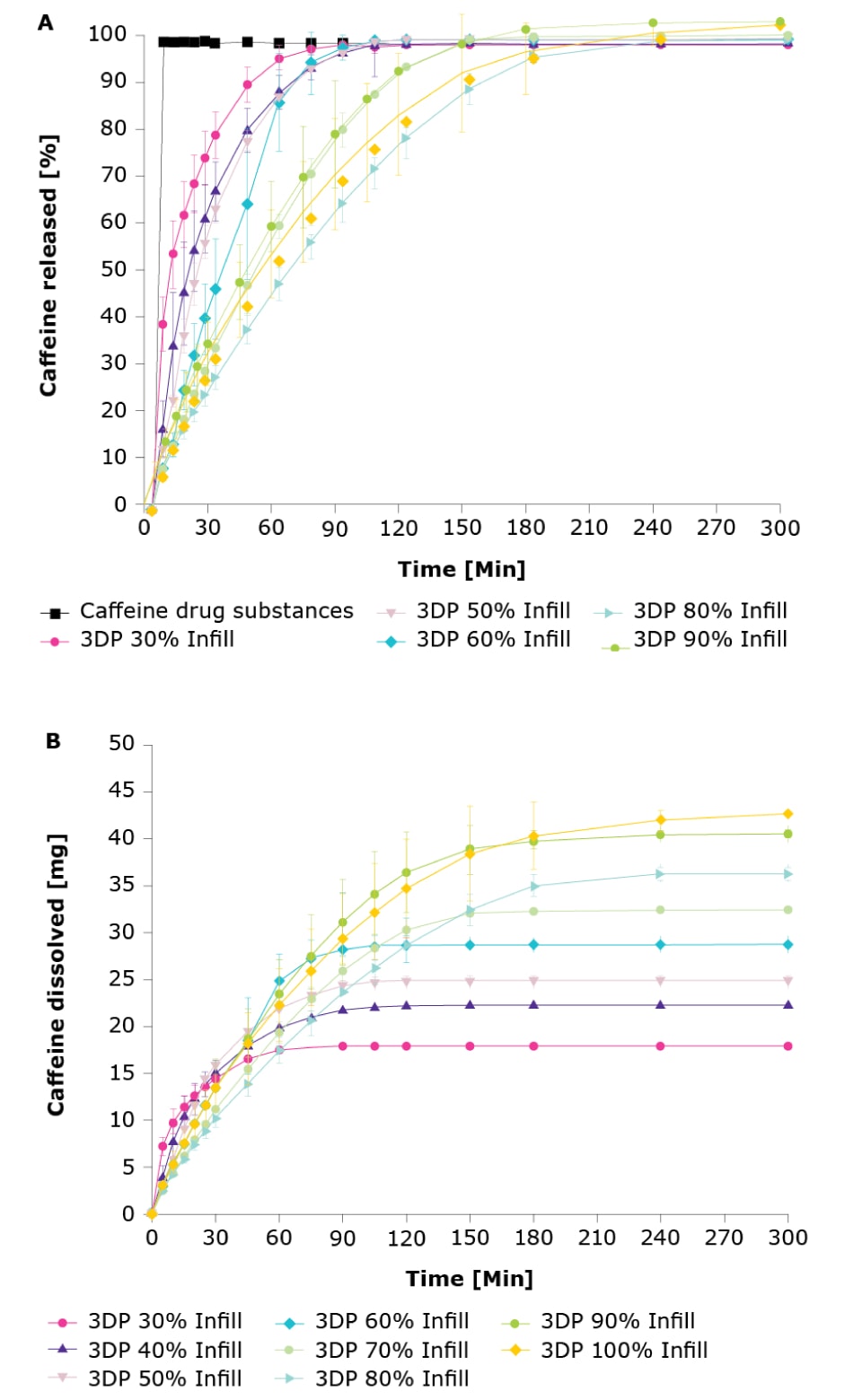
Figure 7.Comparison of drug release kinetics using different infill volumes.
Opportunities from Direct Melting Technologies for 3D Printing Solid Formulation Drugs
Direct melting technologies like melt drop deposition provide very high accuracy for melt-based printing systems as the droplet geometry can be precisely defined. A key differentiator from existing fused deposition modeling (FDM) technologies is that the process itself is based on a single melting step and as such, powder or granules can be used to start the process and a second melting step is not necessary. In addition, there is a very broad processing range linked to direct extrusion and complex forms can be realized due to highly defined material deposition. Independent of the 3D printing technology selected, polymer requirements are a critical success factor. The high thermal stability of the polymer is a key requirement as well as its mechanical properties.
Opportunities for further development of melt-based 3D printing are numerous. The technology can be used to create tablets in a wide variety of shapes and sizes and regulate the number of active substances in the composition of the tablet. This approach also enables the controlled release of the API which can increase the effectiveness of the medication and provides the ability to overcome solubility challenges. 3D printing also has the potential to accelerate pharmaceutical development timelines by rapidly supplying prototypes for clinical trials and at the same time, provides the ability to print small batches of drugs, saving time and money on the establishment of large-scale manufacturing lines.
Related Products
References
To continue reading please sign in or create an account.
Don't Have An Account?