Performing an Isolation of Recombinant Protein Complexes
For structural studies and for comprehensive functional studies of multiprotein complexes it is often necessary to isolate protein amounts in the mg range. As mentioned above, pulldown methods are often not well suited for the isolation of such large protein quantities. An alternative approach is in vitro reconstitution. This can be used to assemble complexes that are subsequently isolated. The examples in this section cover in vitro assembly of complexes by two different recombinant approaches.
Purification of a 145-subunit complex by affinity chromatography
The human spliceosome, a ~145 subunit complex containing both RNA and protein, was purified using affinity chromatography as the main step in an approach that used a recombinant bait protein as a bridge between the complex and the affinity medium.
Spliceosomes were assembled by in vitro reconstitution using a nuclear extract from HeLa cells and a 32P-labeled pre-mRNA. To facilitate the purification, an affinity tagged protein that binds to the labeled pre-mRNA (but is not a component of the spliceosome) was added. The spliceosome was then captured via the tagged protein by affinity chromatography. Final purification was performed using gel filtration (Figure 2.7).

Figure 2.7.Purification of human spliceosomes, a multiprotein complex with more than 140 subunits. From reference 11. A) Purification scheme for human spliceosomes. B) Gel filtration analysis monitored by scintillation counting. Spliceosomes were separated on Sephacryl S-500 before (blue) and after (green) affinity purification c.p.m. = counts per minute. Used with permission. Copyright 2002 Nature Publishing.
Purification of individual overexpressed proteins and in vitro reconstitution of a multiprotein complex
Complexes with a limited number of subunits have been reconstituted in vitro. One example is shown in Figure 2.8. The nucleosome (the nucleoprotein complex of eukaryotic chromatin) consists of an octamer formed by four histone proteins (H2A, H2B, H3 and H4), and 146 base pairs of DNA.
Each of the four different histones were individually overexpressed in E. coli and obtained in inclusion bodies. The inclusion bodies were solubilized in guanidinium HCl and purified under denaturing conditions. The purified, denatured histones were then mixed, and refolding and reconstitution were performed by dialysis. The histones spontaneously folded into soluble octamers. Remaining aggregates were removed by gel filtration (Superose 12). The 146-bp DNA was added, and the multiprotein complex (the nucleosome core particle) was spontaneously assembled. The complex was then crystallized for 3-D structure determination.
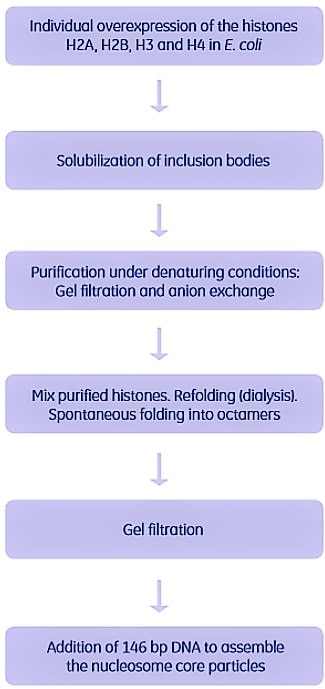
Figure 2.8.Purification of the individual subunits and in vitro reconstitution of the nucleosome. Used with permission. Copyright 1997 Nature Publishing.
Another example involves the study of regulatory factor complex (RFX complex), a subcomplex of the major histocompatibility complex class II (MHCII) enhanceosome. The DNA binding RFX complex is required for activation of MHCII gene expression. The RFX complex is composed of three proteins, RFXB, RFXAP and RFX5. The RFX5 protein is identified to have five domains and the N-terminal domain (1-90) is responsible for oligomerization.
In this study, RFXB and constructs of different lengths of RFX5 were expressed as histidine-tagged proteins and purified as shown in Figure 2.9. For RFXAP, as it formed a stable complex with the N terminal domain of RFX5, it was copurified with RFX5 (1-90). Cells containing these two overexpressed proteins were lysed together, and purification of the complex of two proteins was performed using IMAC. The complex of two proteins was subjected to a second IMAC step under denaturing conditions. RFXAP was recovered in the flowthrough, refolded, concentrated and further purified by gel filtration.
The purified proteins and protein fragments were used in oligomerization studies to develop a DNA binding model for the interaction between the RFX complex and the MHCII promoter.
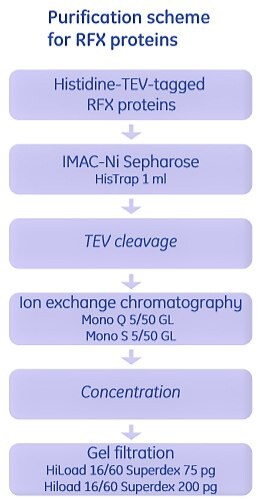
Figure 2.9.Purification of the RFX complex proteins of the MHCII enhanceosome. The RFX complex is composed of three proteins RFXB, RFXAP, and RFX5 that associate in a 1:1:2 or 1:1:4 ratio, respectively. Full-length RFXB and RFXAP, and protein fragments of RFX5 were overexpressed and purified using IMAC, ion exchange chromatography and gel filtration.
Materials
To continue reading please sign in or create an account.
Don't Have An Account?