Chemoselective Purification Tags
Introduction
Whilst RP-HPLC is an extremely powerful tool for the purification of small to medium sized peptides, for long peptides the technique lacks the resolution necessary to separate the target molecule from the melange of closely related deletion and truncation products that arise during synthesis. In addition, the purified products, despite giving the appearance of being homogeneous by HPLC analysis, are often microheterogeneous, being contaminated with numerous co-eluting sequences which, because they are individually only present in small amounts, escape detection by mass spectrometry.
One solution is to utilize a combination of chemoselective purification tags1 and standard RP-HPLC. In the former, unreacted amino groups are capped after each coupling step, converting deletion sequences to shorter truncation sequences. Prior to cleavage of the peptide from the resin, the N-terminal amino functionality of the full-length peptide is labeled with an affinity tag which permits selective separation of the tagged target peptide from these truncation sequences. Following affinity purification, the tag is cleaved and the desired peptide isolated. The method is especially effective at removing impurities that are closely eluting or hidden under the isolated product peak. Furthermore, removal of these ion-suppressing smaller impurities can greatly enhance the signal of the target peptide in ES-MS. Final polishing by HPLC, removes modified and partially-protected by-products.
Novabiochem® offers two chemoselective purification tags: IMAC Tag and 2-biotinyldimedone.
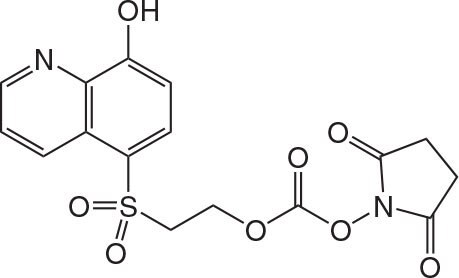
Figure 1.IMAC Tag
The mechanism of purification using the IMAC Tag2 is analogous to HisTag affinity purification, the traditional method in use for isolation and purification of recombinant proteins. The IMAC purification method is extremely easy-to-use, gives higher recoveries than RP-HPLC, and is more effective at removing closely eluting impurities. Furthermore, as the purification is an on-off process, it can be readily automated using standard HPLC or FPLC instrumentation.
The peptide is synthesized using standard methods. After each coupling unreacted amino groups are capped using Ac2O or Z(Cl)-OSu. Once the final Fmoc group is removed, the IMAC tag is attached to the peptide via an ONp carbonate (Figure 5-36). Peptides are then cleaved from the resin under standard conditions and purified using a column functionalised with iminodiacetic acid (IDA) loaded with Cu2+ ions. Tagged peptide is bound to the column at pH 6.5 – 8.0 and truncated sequences are washed away before the tagged peptide is eluted from the column by adjusting the pH to 3.5 (Figure 3). Buffers incorporating sodium phosphate, sodium chloride and urea are used to ensure maximum solubility of substrates.
Once the purified tagged peptide is eluted from the column, the TAG can be removed by simply raising the pH to 11.0 using 2 M NaOH for a short period. The liberated TAG and purified peptide can then be separated using the same IMAC column or an HPLC column for combined isolation and de-salting. As reducing agents are not compatible with IDA, any cysteines present in peptides must be protected during the IMAC step. This can be done simply and reversibly using for example StBu protection.
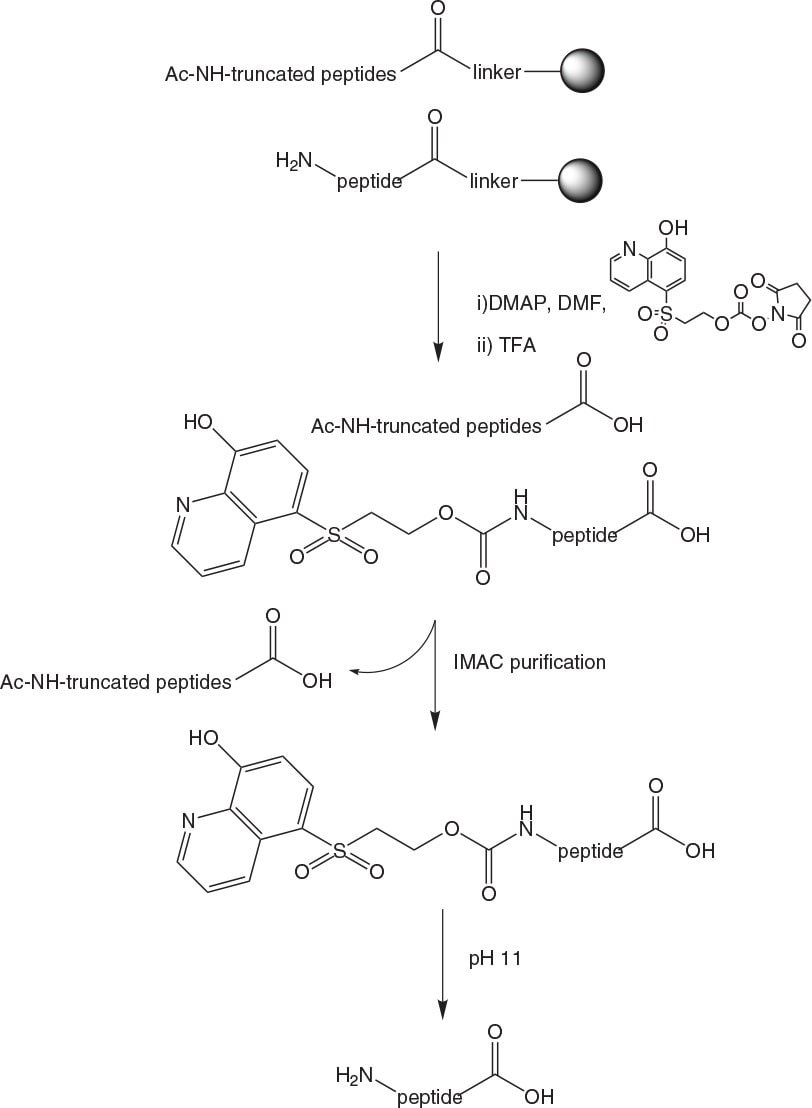
Figure 2.IMAC purification of peptides
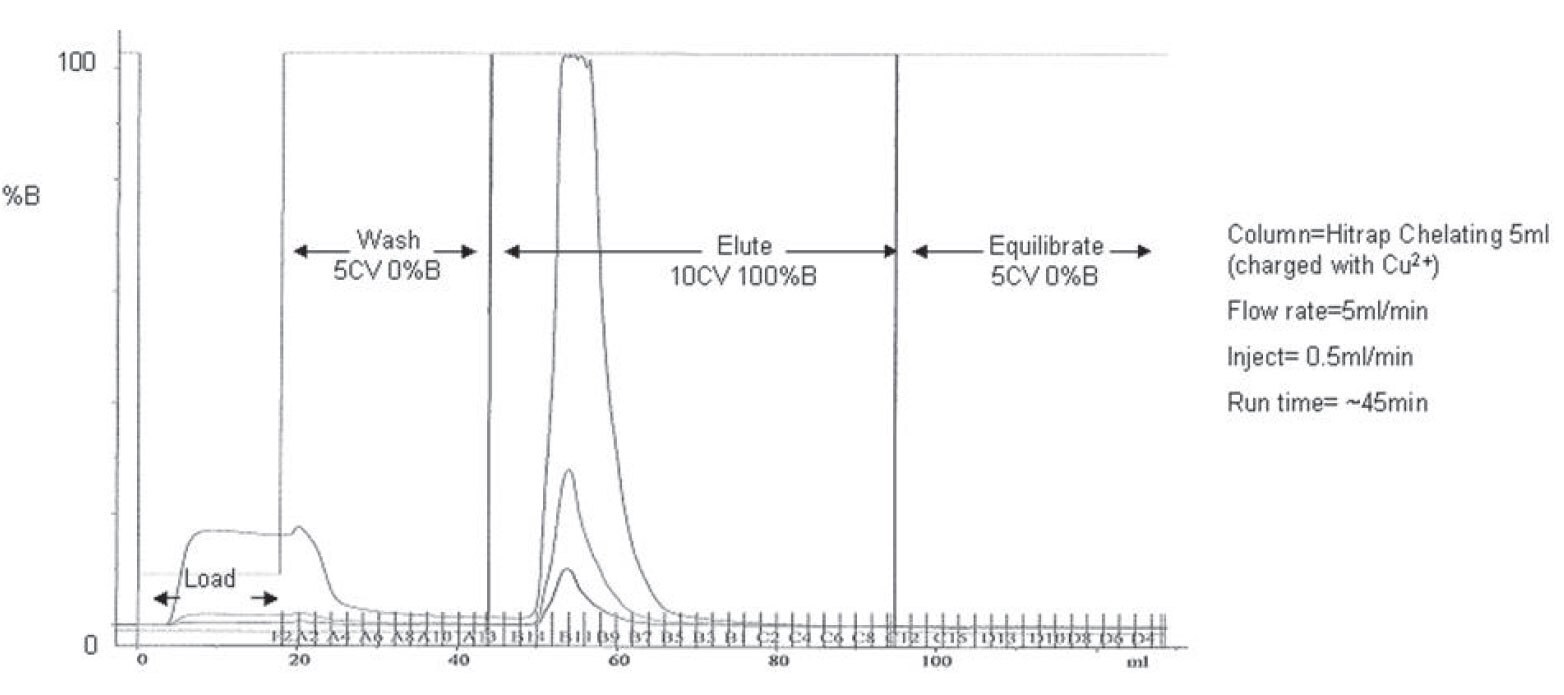
Figure 3.A typical IMAC purification trace obtained using a GE Akta Explorer purification system. Untagged truncates elute during the load and wash step and tagged full length product remains bound to the column until the elute step.
Protocols
The procedure of attachment of the IMAC and cleavage of tagged peptide from the resin are shown in Methods 1-3.
Method 1: Formation of IMAC-tagged peptide
Attachment of IMAC tag
- Dissolve Tag-OSu in the minimum volume of DMF and add to pre-swollen resin. Coupling has been shown to be effective using as little as 1 eq Tag-OSu with respect to the initial resin loading.
- Agitate the mixture for one hour, add a catalytic amount of DMAP and agitate for a further hour.
- Wash the resin with DMF, DCM and diethyl ether before drying under vacuum.
Cleavage of tagged peptide from the resin
- Cleave non-cysteine containing peptides from the solid phase with concomitant side-chain deprotection by treatment with 90%TFA v/v, 5% H2O v/v, 2.5% TIS v/v, 2.5% EDT v/v for 4.5 hours.
- Cleave peptide-resins containing Cys(StBu) by treatment with 90% TFA v/v, 5% H2O v/v, 5% TIS v/v for 4.5 hours.
- Work up cleavage reactions in the standard manner by precipitation.
The IMAC purification using a copper (II) loaded IDA column is given in Method 2. Columns such as HiTrap Chelating HP worked well for this application.
Method 2: Purification of tagged peptide
Buffer preparation
- Binding buffer for the IMAC chromatography may consist of 20 mM sodium phosphate, 2-8 M urea (depending on peptide solubility) and 0.5 M NaCl. A pH range of 6.5-8.5 can be used.
- Elution Buffer should have a pH of 3.5. A suggested buffer within this range is citric acid-sodium phosphate system. Elution buffer should also contain Urea (2-8 M) and NaCl (0.5 M).
IMAC purification
- Charge column with 0.5 column volumes (CV) of 0.1 M CuSO4 in H2O.
- Wash column with 2 CV H2O, 7.5 CV elution buffer, 10 CV binding buffer.
- Solubilise crude tagged peptide in binding buffer.
- Load sample onto IMAC column.
- Wash any unbound material from the column with 10 CV binding buffer.
- Elute tagged peptide from column using 20 CV elution buffer.
Method 3: Removal of tag
- fractions containing purified tagged product. Adjust to pH 11 with 2 M NaOH. If product contains Cys(StBu) add TCEP up to a concentration of 5 mM to remove S-tBu protecting groups simultaneously.
- Agitate reaction for 1-2 hours at room temperature and then adjust pH to ~ 4 with 2 M HCl.
- Separate liberated tag and purified peptide using a second IMAC step or carry out RP-HPLC to combine isolation with desalting.
Purification of Ubiquitin
Ubiquitin was also tagged and purified using IMAC methodology. The crude peptide was shown to contain several impurities identified as capped truncates formed during SPPS (Figure 4). A recovery of 62% pure peptide (89% purity) was obtained after IMAC purification and TAG cleavage. For comparison, purification using HPLC gave pure peptide (82%) with a lower recovery of 21%.
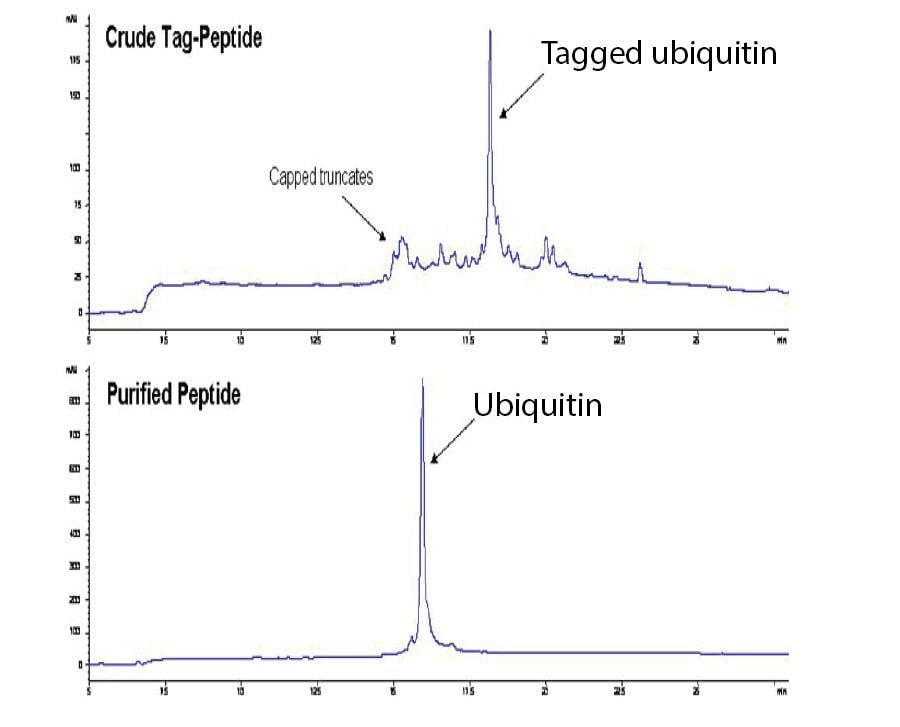
Figure 4.HPLC profiles of ubiquitin before and after IMAC purification
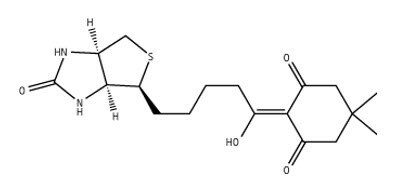
Figure 5.2-Biotinyldimedone
2-Biotinyldimedone tag allows product isolation by biotin-avidin affinity chromatography4. Without any pre-activation, it reacts with free primary amino groups to give derivatives that are stable to both acid and base conditions employed in Fmoc SPPS. Labeling of the N-terminal amine of resin-bound peptides is simply carried out by incubating overnight the peptidyl resin with a fourfold excess of the reagent in DMF. The biotinylated peptide is obtained following the standard TFA cleavage-deprotection procedure. Following cleavage, this biotin-labeled peptide can be bound to an avidin-coated support, allowing capped fragments to be simply washed away. The purified product can then be eluted with aqueous hydrazine, leaving the used tag attached to the support.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?