Cross-Coupling

A cross-coupling reaction in organic synthesis occurs when two fragments are joined together with the aid of a metal catalyst. Cross-coupling has been an essential reaction in catalytic chemistry for the past 30 years starting with the pioneering work by Heck, Negishi, and Suzuki, who were awarded the Nobel Prize in Chemistry in 2010 for palladium-catalyzed cross-coupling. As one of the most versatile and powerful bond-forming methods in synthetic organic chemistry, the field of cross-coupling has matured to the point where nearly any two fragments can be coupled with the right catalyst.
Featured Categories
We’re proud to offer a comprehensive portfolio of heterocyclic building blocks, one of the largest and most diverse families of molecular fragments used in organic synthesis.
We offer an extensive portfolio of nickel and palladium catalysts to aid in the creation of C–C, C–N, and C–O bonds through common cross-coupling reactions.
Find the basic components needed to drive your research forward in our portfolio of organic building blocks. Alkenesm alkanes, alkynes, arenes, allenes & more!
Discover our unparalleled portfolio of transition metal catalysts for your different organic and organometallic chemistry-based applications.
With its usage increasing exponentially, the field has grown to include numerous strategies for carbon-carbon, carbon-nitrogen, and carbon-oxygen bond formation, including key reactions such as:
- Buchwald-Hartwig amination, the cross-coupling of an aryl (pseudo)halide and an amine, is a staple reaction for chemists across a wide range of disciplines.
- Heck coupling is the cross coupling of an unsaturated halide with an alkene to give substituted alkenes.
- Negishi coupling is the cross-coupling of an aryl (pseudo)halide and an organozinc nucleophile to form C-C bonds.
- Sonogashira coupling is the cross-coupling of an aryl (pseudo) halide with a terminal alkyne to give disubstituted acetylenes.
- Stille coupling, the cross-coupling of an aryl (pseudo)halide and stannane, is a functional reaction for carbon-carbon bond formation with few limitations on the R-groups.
- Suzuki-Miyaura coupling, the cross-coupling of an aryl (pseudo)halide and organoborate, is a versatile reaction for carbon-carbon bond formation.
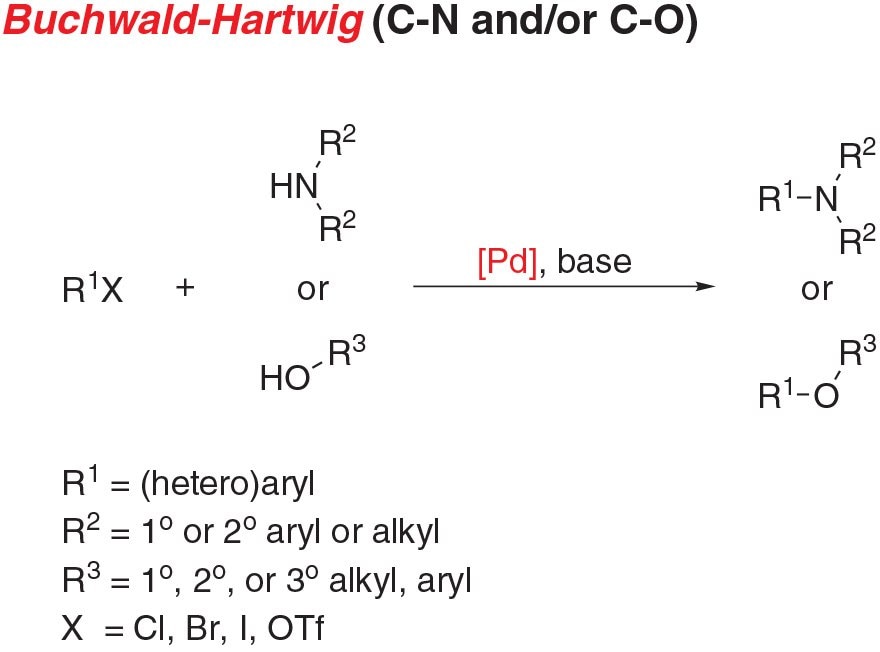
Figure 1.Buchwald-Hartwig Amination

Figure 2.Heck Cross-Coupling Reaction
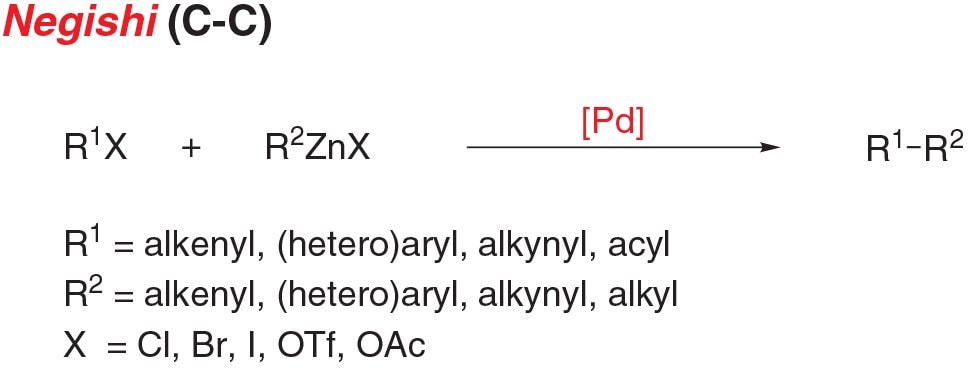
Figure 3.Negishi Cross-Coupling Reaction
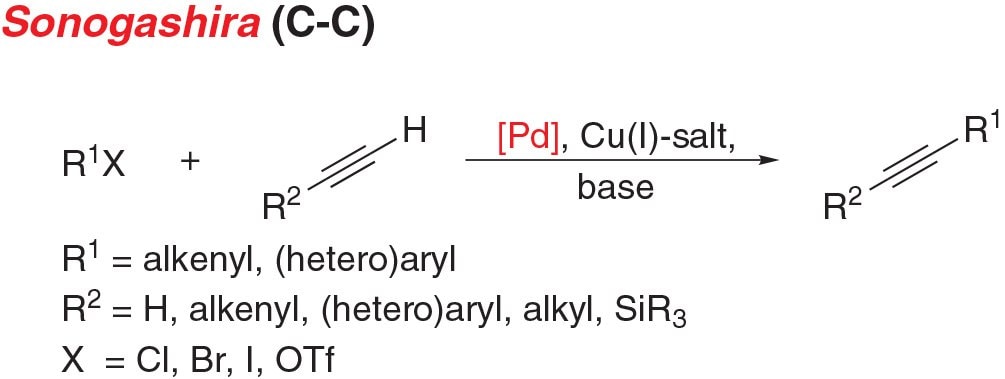
Figure 4.Sonogashira Cross-Coupling Reaction
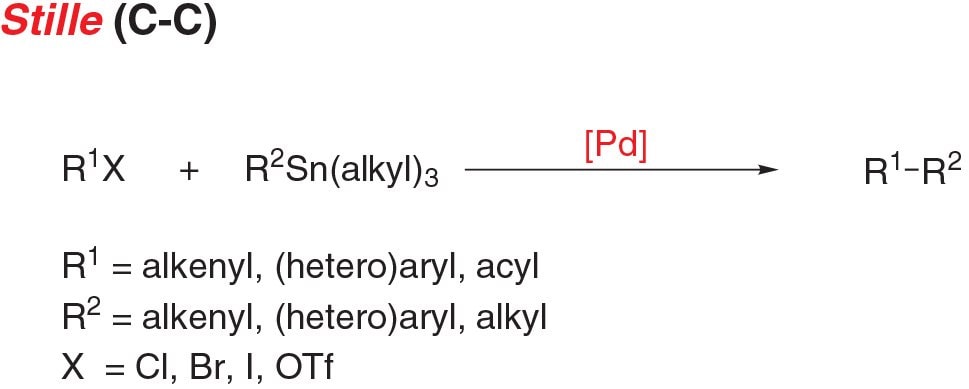
Figure 5.Stille Cross-Coupling Reaction

Figure 6.Suzuki-Miyaura Cross-Coupling Reaction
Visit our document search for data sheets, certificates and technical documentation.
Related Articles
- G3 and G4 Buchwald palladium precatalysts are the newest air, moisture, and thermally stable crossing-coupling complexes used in bond formation for their versatility and high reactivity.
- MIDA-protected boronate esters offer stability, chromatography compatibility, and reactivity in anhydrous cross-coupling conditions.
- Lipshutz and co-workers have recently developed a second generation technology to their original PTS-enabling surfactant based on the polyoxyethanyl-α-tocopheryl succinate derivative, TPGS-750-M.
- C2-symmetric chiral bisoxazolines (BOX) are privileged structures because they promote a great number of transformations with unprecedented selectivity.1
- The Pd-catalyzed C–N bond formation has become an important synthetic reaction in the past 20 years. Several research groups have investigated this reaction and developed very versatile catalysts. Buchwald and coworkers have been very active in synthesizing and developing a portfolio of phosphine ligands for this transformation and other cross-coupling reactions.
- See All (83)
Related Protocols
- Protocol for the KitAlysis™ Buchwald-Hartwig Amination Reaction Screening Kit
- Solutions & slurries to make: Go to the online user set-up page to enter molecular weights into the downloadable excel file.
- TPGS-750-M surfactant enables various reactions in water at room temperature, enhancing efficiency and versatility in synthesis.
- KitAlysis™ Cu C-N (Buchwald-Hartwig) cross-coupling high-throughput screening kit. Detailed Set-Up User Guide and a downloadable excel file for calculations.
- A step-by-step protocol guide for KitAlysis Suzuki-Miyaura Cross-Coupling Reaction Screening Kit.
- See All (6)
How Can We Help
In case of any questions, please submit a customer support request
or talk to our customer service team:
Email custserv@sial.com
or call +1 (800) 244-1173
Additional Support
- Chromatogram Search
Use the Chromatogram Search to identify unknown compounds in your sample.
- Calculators & Apps
Web Toolbox - science research tools and resources for analytical chemistry, life science, chemical synthesis and materials science.
- Customer Support Request
Customer support including help with orders, products, accounts, and website technical issues.
- FAQ
Explore our Frequently Asked Questions for answers to commonly asked questions about our products and services.
To continue reading please sign in or create an account.
Don't Have An Account?