Mycoplasma Testing in Pharma
Mycoplasma contamination is a widespread and reoccurring problem for many cell culture systems in life science research and the pharmaceutical industry. Mycoplasmas can grow to high titers in culture media without exhibiting typical bacterial contamination signs such as turbidity. Their effects on cultured cells include altered metabolism, slowed proliferation, and chromosomal aberrations. In short, Mycoplasma contamination compromises the data validity of affected cell lines and of results in life science research. This makes Mycoplasma testing and thus the maintenance of contamination-free cell cultures fundamental to cell-based research and to the manufacturing of goods where consumer health is a major concern.
Featured Categories
A microbial culture medium is a mixture of substances that promotes and supports the growth and differentiation of microorganisms.
Sterility testing is an essential part of validation for products manufactured according to GMP purporting to be sterile.
Ensure safety: Pyrogen testing is vital for pharmaceuticals, devices. Monocyte Activation Test (MAT) detects endotoxin, non-endotoxin pyrogens.
Discover advanced environmental monitoring and aseptic process simulation solutions for pharmaceutical manufacturing. Ensure GMP compliance with our specialized tools and media for cleanrooms, isolators, and RABS.

BIOSAFETY TESTING SERVICES
Biosafety testing services to investigate mAbs and cell and gene therapy and vaccines materials to ensure that they are free of adventitious agents or unexpected results that can cause catastrophic failures in downstream processing, including: Mycoplasma testing.
About Mycoplasma Bacteria
Mycoplasmas are among the smallest known bacteria, being able to pass filters with pore sizes of 0.2 µm. These microorganisms grow aerobically or under facultative anaerobic conditions.
Mycoplasmas are either parasitic or saprophytic. Several species, for example M. pneumoniae, are pathogenic, causing pneumonia and other respiratory disorders in humans. M. genitalium is presumably involved in pelvic inflammatory diseases. As bacteria that lack a cell wall, mycoplasmas are not susceptible to penicillins or other antibiotics that act on this structure. To stabilize their cytoplasmic membranes most mycoplasmas require sterols, which they take up from their environment, usually as cholesterol from their animal hosts. Many Mycoplasma strains are also tolerant to a variety of other antibiotics.
Visit our document search for data sheets, certificates and technical documentation.
Sources of Mycoplasma Contamination
The sources of Mycoplasma contamination in the laboratory and in manufacturing are very challenging to control. Some species are found on human skin and can be introduced to cultures through poor aseptic practices. Additionally, mycoplasmas may be introduced via contaminated supplements, such as fetal bovine serum or, most commonly, by transmission from other contaminated cell cultures. Once a cell culture contains mycoplasmas, these microbes can quickly spread and contaminate other areas of the lab by aerosols and particulates generated during culture handling. Strict adherence to good aseptic laboratory practices is key, and routine testing of cultures is highly recommended for successful control of mycoplasma contamination in manufacturing areas.
Why Mycoplasma Testing in the Pharmaceutical Industry?
Biopharmaceutical facilities that use eukaryotic cells for the production of vaccines have to test their cell banks and virus seed lots as well as bulk vaccines for contamination with Mycoplasma. Species from this bacterial genus infect eukaryotic cells, disrupting their growth and metabolism. When mycoplasmas interfere with vaccine production, this may affect protein quality and yields, and more importantly cause side effects in patients who are administered the final vaccine product.
Bioreactor contamination with mycoplasmas can lead to significant loss of time, materials and revenue unless identified early in the manufacturing process. Without rigorous testing routines, Mycoplasma contamination of biopharmaceutical production batches is extremely difficult to discover because the presence of mycoplasmas does not generally lead to pH changes or visual turbidity in the culture media. The compendial Mycoplasma test method involves both culture and indicator cell tests for detection. If only two media are used for analysis, it is recommended to use FREY and FRIIS media in combination.
Mycoplasma Testing Methods
It is important to test biopharmaceuticals, vaccines, cell cultures and virus cultures for mycoplasmas at various points during quality control. The three most common test methods are:
- Mycoplasma culture, where bacterial culture media optimized for mycoplasmas is inoculated with test samples
- DNA staining methods, where mycoplasma nuclei are counterstained with Hoechst or DAPI and imaged using fluorescence microscopy
- PCR (polymerase chain reaction) to amplify the bacterial DNA if mycoplasmas are present in the sample
Workflow to Detect Mycoplasmas acc. to EP 2.6.7 & USP 63
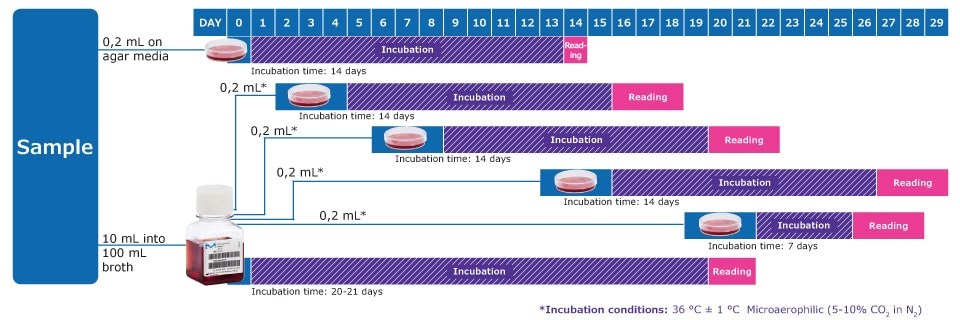
* On days after inoculation, subculture each liquid medium by inoculating 0,2 mL on at least 1 plate of each solid medium. Repeat the procedure between the 6th and 8th day, again between the 13th and 15th day and again between the 19th and 21st day of the test. Observe the liquid media every 2 or 3 days and if a color change occurs, subculture.
Note: In addition, one positive and one negative control per solid and liquid medium is required, with an incubation time of 14 days.
Ready-to-Use Media for Compendial Mycoplasma Detection
We provide a complete portfolio of ready-to-use liquid and solid culture media required for detecting mycoplasmas according to European Pharmacopoeia 6.1 (2.6.7.) and USP 35 (63). Without any further preparation steps, our ready-to-use Mycoplasma culture media ensure constant product quality and complete traceability with lot specific certificates of analyses.
Due to their ingredients, Mycoplasma media generally have far shorter shelf lives than most other culture media for microbiological quality control. The shelf lives of our ready-to-use Friis, Frey, and Hayflick liquid and solid media have been extended to 4 months for the agar plates and 6 months for the broths. Experience our complete range of ready-to-use culture media with a longer shelf life to make Mycoplasma testing easier.
Related Articles
- Cell culture protocol for testing cell lines for mycoplasma contamination using indirect DNA staining using Hoechst 33342.
- See All (1)
Find More Articles
How Can We Help
In case of any questions, please submit a customer support request
or talk to our customer service team:
Email custserv@sial.com
or call +1 (800) 244-1173
Additional Support
- Chromatogram Search
Use the Chromatogram Search to identify unknown compounds in your sample.
- Calculators & Apps
Web Toolbox - science research tools and resources for analytical chemistry, life science, chemical synthesis and materials science.
- Customer Support Request
Customer support including help with orders, products, accounts, and website technical issues.
- FAQ
Explore our Frequently Asked Questions for answers to commonly asked questions about our products and services.
To continue reading please sign in or create an account.
Don't Have An Account?