Gene Therapy Manufacturing
Gene therapies offer an unprecedented potential to treat and cure countless diseases and conditions for which patients have no or limited treatment options. Hundreds of gene therapies are currently in development around the world, being advanced by both early-stage companies and established industry leaders.
Successful development of a gene therapy requires expertise that differs from the production of other biologics such as monoclonal antibodies. Viral vector manufacturing and specialized testing capabilities are unique to this modality and just two areas that can determine the success of a candidate gene therapy. The lack of established process templates, evolving regulatory guidelines, and the need to meet accelerated timelines put additional pressure on gene therapy manufacturers to get it right the first time.
Given the remarkable opportunities and challenges presented by gene therapies, alliances with experienced technology partners or contract development and manufacturing organizations (CDMOs) are essential to ensure successful process development, scale-up, manufacturing, and regulatory compliance.
Reach out to us for more details on our bioprocessing materials and technologies for viral vector production and an expert will respond back to you shortly.
Featured Categories
Our off-the-shelf and customizable bioprocessing cell culture media (CCM) products enhance productivity in upstream mAb, vaccine, gene/cell therapy processes.
Cell culture media filtration prevents bioreactor contamination. Selection of a sterilizing filter, mycoplasma filter, or virus filter depends on process risk. Learn how to select the best filter for your cell culture media filtration.
Upstream process chemicals ensure superior performance of your cell culture process driving cell viability, productivity and critical quality attributes of the drug product.
Browse our high-quality excipients, with solutions for solid, liquid, semisolid, and biomolecule formulation, as well as advanced drug delivery.
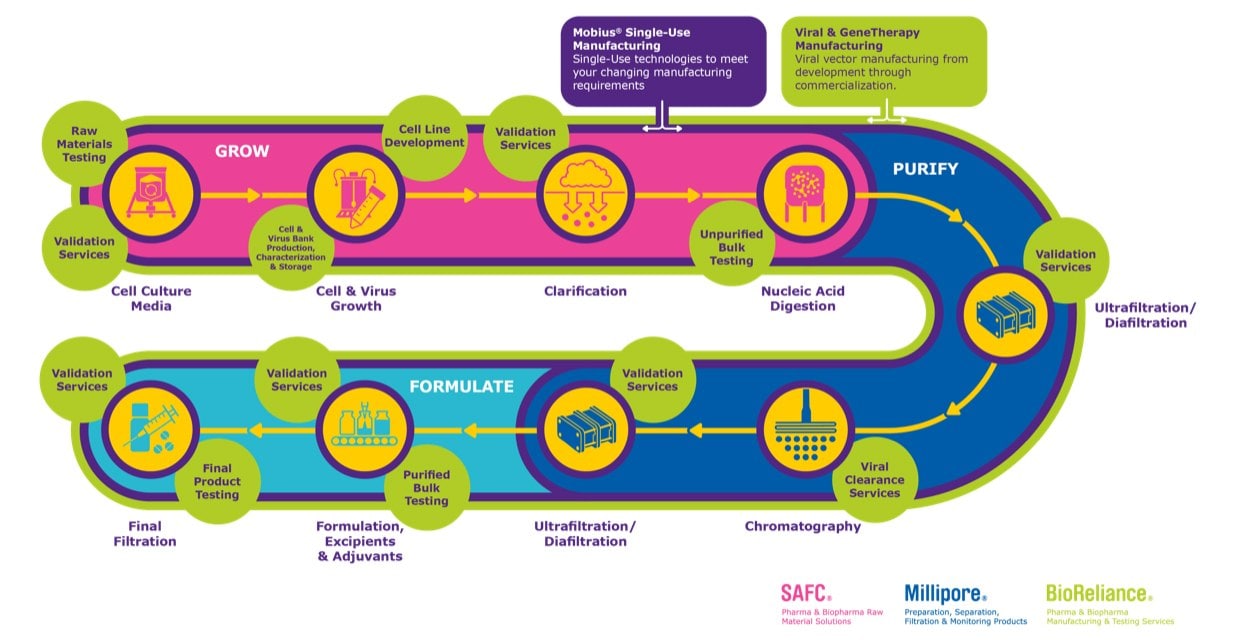
Gene therapy development: Grow, purify, formulate
Visit our document search for data sheets, certificates and technical documentation.
Workflow
Viral Vector Downstream Processing
Efficient virus purification processes can improve yield, decrease time to patient, and lower manufacturing costs
Viral Vector Formulation and Final Fill
Formulating a commercially viable gene therapy demands a high level of application and regulatory expertise
Viral Vector Characterization and Biosafety Testing
Critical biosafety testing and characterization of viral vector products can help to fully analyze key quality attributes: identity, potency, safety, and stability
Viral Vector Contract Development and Manufacturing
CDMO partnerships play a critical role in advancing clinical pipelines and achieving successful commercialization
Reach out to us for more details on our bioprocessing materials and technologies for viral vector production and an expert will respond back to you shortly.
Related Technical Articles
- This page describes key considerations for cell lysis and how the combination of a high salt concentration and a salt tolerant endonuclease can be used to increase vector titer and infectivity during AAV vector manufacturing.
- Learn how development of a suspension adapted HEK293T cell line with accompanying cell culture media can maximize lentivirus production for cell and gene therapies.
- Learn how the VirusExpress® lentivirus platform utilizes DoE experiments and supplementary studies to optimize workflows, enhance yield, and ensure quality in cell and gene therapies.
- Explore lentivirus harvest clarification options for viral therapy production using suspension cell lines in manufacturing platform development.
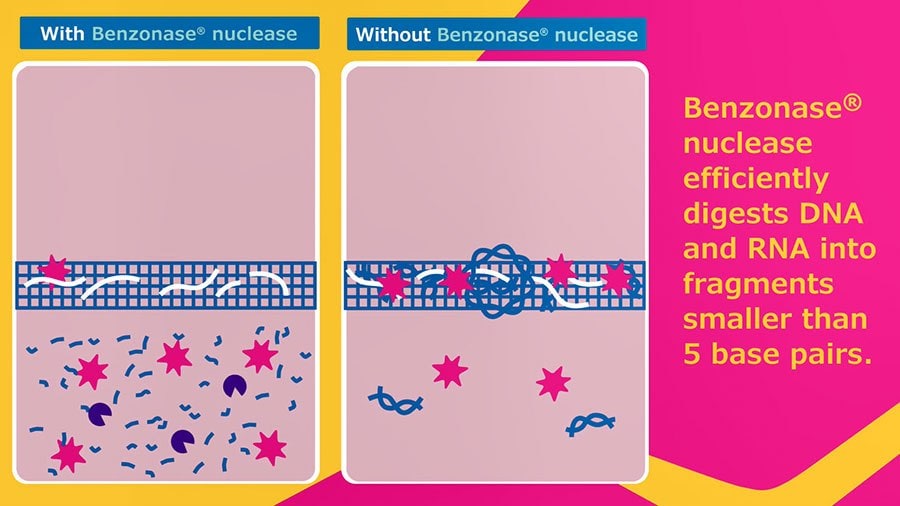
- Benzonase®endonuclease efficiently removes nucleic acid contaminants from viral production, crucial for cell and gene therapies and vaccines.
- See All (11)
Find More Articles and Protocols
Related Resources
- Product Guide: Upstream Chemicals for Bioprocessing
Our comprehensive portfolio of upstream process chemicals not only provides biopharmaceutical manufacturers with high-quality raw materials for classic and novel therapies, but also helps them get to market faster and simplify regulatory challenges.
- Brochure: Draw on Our Experience: Gene Therapy Capabilities for AAV and Lentivirus Production
You’ve created a gene therapy with the possibility to help patients. But the development and manufacturing of your gene therapy, how it’s developed, produced and approved, are still being outlined.
- Application Note: One Size Does Not Fit All: Utilizing Multiple Technology Provideers in Gene Therapy Manufacturing
Despite notable clinical and even commercial success, the demand for gene therapies still outweighs supply.
- Technical Bulletin: Regionalizing and Democratizing Cell and Gene Therapy
How MilliporeSigma and SaudiVax are working together to help patients in the Middle East and North Africa access the benefits of advanced medicine.
- Application Note: Viral Vectors: Are We There Yet?
Looking at the current state of cell and gene therapies (CGTs), it is clear they have brought major changes to the biopharmaceutical landscape.
- Application Note: Perspectives Of Value-Driven, Integrated Solutions In Gene Therapy
With rapid market growth and inspiring stories of success, gene therapies boast a promising future for manufacturers and patients alike.
- Brochure: Process Development and Drug Manufacturing: Support Services
We provide comprehensive services for drug development and manufacturing, including technical and regulatory expertise and process development support.
How Can We Help
In case of any questions, please submit a customer support request
or talk to our customer service team:
Email custserv@sial.com
or call +1 (800) 244-1173
Additional Support
- Chromatogram Search
Use the Chromatogram Search to identify unknown compounds in your sample.
- Calculators & Apps
Web Toolbox - science research tools and resources for analytical chemistry, life science, chemical synthesis and materials science.
- Customer Support Request
Customer support including help with orders, products, accounts, and website technical issues.
- FAQ
Explore our Frequently Asked Questions for answers to commonly asked questions about our products and services.
To continue reading please sign in or create an account.
Don't Have An Account?