AAV Process Intensification Using High Salt Lysis and Salt Tolerant Endonuclease
This page describes key considerations for cell lysis and how the combination of a high salt concentration and a salt-tolerant endonuclease can be used to increase vector titer and infectivity.

Production of adeno-associated virus (AAV) vectors includes a midstream step that combines cell lysis and nuclease treatment. Here, detergents are used to break apart the lipid bilayer of the cells, releasing the vectors, while host cell DNA and remaining plasmids are digested with an endonuclease to ensure patient safety and improve downstream process efficiency. Residual DNA must be reduced to less than 10 ng/dose and the fragment size must be less than approximately 200 base pairs as recommended in the 2020 FDA Guidance for Gene Therapy Investigational New Drug Applications.
Considerations for Cell Lysis
While seemingly straightforward, the cell lysis step presents several challenges. Commonly used detergents for cell lysis, such as TRITON™ X-100 (4-tert-octylphenol polyethoxylate), can be problematic. As of January 2021, the unauthorized use of TRITON™ X-100 has been prohibited in the European Union by the European Commission due to its listing on REACH (Registration, Evaluation, Authorization and Restriction of Chemicals). This listing, along with strict guidelines related to its use in the EU, are due to the endocrine and mutagenic effects of the degradation product of TRITON™ X-100 which has been determined to be a danger to patients and the environment.
Additional considerations when selecting a detergent for cell lysis is that it should not damage the viral vectors and viral particle infectivity, and it should not interfere with the downstream process, which can occur with use of polysorbates. The selected detergent must also be amendable to removal and detection in subsequent workflow steps.
Finally, it is important to understand the impact of salt concentration on cell lysis and vector yield. Historically, lysis buffers used for release of AAV capsids contained a physiological concentration of salt (150mM NaCl). Recent publications, however, have reported that increasing the salt concentration to 500mM increases the number of vector particles and infectious titers and reduces AAV aggregation. As described below, however, high salt can negatively impact the activity of conventional endonucleases used for DNA digestion in the AAV vector manufacturing process.
Effect of High Salinity During Midstream Steps on AAV Yield
A study was conducted to determine the impact of a higher salt concentration during cell lysis. AAV5 vectors were produced in HEK293 cells which were lysed with either TRITON™ X-100, polysorbate 20, Deviron® C16, or Deviron® 13-S9. The baseline number of capsids produced by a process was determined using 150mM NaCl in a polysorbate 20 lysis buffer. Use of 500mM salt in the lysis buffer led to an increase in AAV5 capsid titers by an average of 29% (Figure 1).
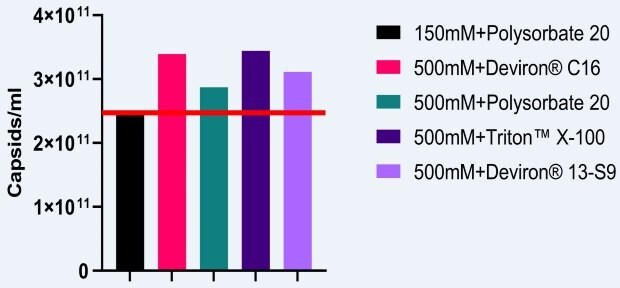
Figure 1.Impact of high salt lysis on the production of AAV5 titers.
Effect of High Salt Concentration During Midstream Steps on AAV Infectivity
To measure the effect of the 500mM NaCl conditions on AAV infectivity, a similar study was performed in which AAV2 vectors were manufactured in HEK293 cells. Baseline lysis buffer conditions were 150mM NaCl and polysorbate 20. When the salt concentration was increased to 500mM and different detergents were used, an increase of infectivity of at least 10x was observed (Figure 2). As noted above, an additional benefit of using a high salt concentration during lysis is the reduction in AAV aggregation.
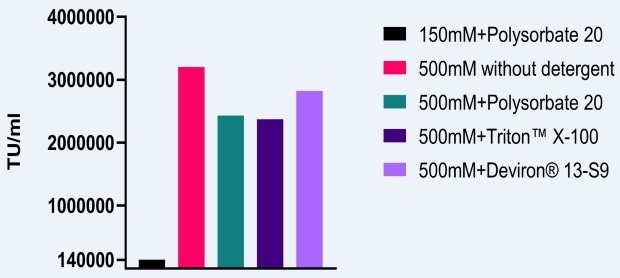
Figure 2.Impact of high salt lysis on AAV5 infectivity.
Considerations for DNA Digestion
As with the cell lysis step, DNA digestion also presents several challenges. Among the most important is the need to source materials with the appropriate quality to meet regulatory requirements and ensure supply chain robustness. In addition to being GMP, purity, glycosylation status, and bioburden are among the factors to be considered. Tolerance of the enzyme to high salinity has also been a critical factor preventing the use of high salt concentrations during AAV manufacturing.
Key Criteria for Enzyme Selection for AAV Manufacturing
- IPEC PQG GMP or equivalent available, FDA DMF/BBMF
- >99% Pure product
- Devoid of Post Translational modifications
- Mycoplasma Test
- Adventitious viruses Test
- Endotoxin Test
- Tailgate samples availability
- Strong logistic & Supply Robustness
- Technical support for application related questions
- Precise detection method
The enzyme used for DNA digestion must also be effectively removed during downstream processing and its activity at the salt concentration used in the process must be considered. While a higher salt concentration can improve vector yield, the interaction between conventional endonuclease enzymes and the DNA does not happen at high ionic strength, thus preventing cleavage of the nucleic acid. This meant that historically, the desired nuclease activity and the salt concentration had to be appropriately balanced.

Development of a Salt Tolerant Endonuclease for AAV Vector Production
To address the need for an endonuclease with efficient activity at the high salt concentrations preferred for cell lysis, we used advanced protein engineering capabilities to develop the Benzonase® Salt Tolerant endonuclease. This non-animal origin endonuclease can digest DNA and RNA at salt concentrations up to 1000mM and is released as IPEC, PQG, GMP, and as an Emprove® Expert product.
A bacterial expression system is used to produce Benzonase® Salt Tolerant endonuclease as it ensures a precisely defined molecular size, no post-translational modifications, and delivers high batch-to-batch reproducibility. The following data demonstrate the activity and advantages of Benzonase® Salt Tolerant endonuclease.
Protein Profile
A bacterial expression system is used to produce Benzonase® Salt Tolerant endonuclease as it ensures a precisely defined molecular size, no post-translational modifications, and delivers high batch-to-batch reproducibility. The homogeneous profile of the protein allows for an accurate detection via immunoassays (Like ELISA), unlike enzymes expressed in yeast which are heavily glycosylated and have a high batch to batch post translational variability profile.
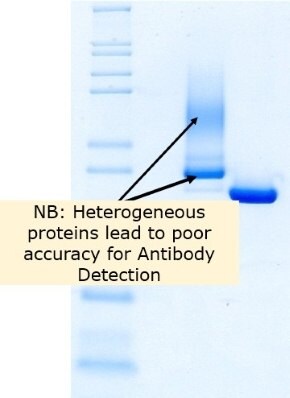
Figure 3.Identity and purity of Benzonase® Salt Tolerant Endonuclease and competitor A as shown by reducing SDS-PAGE.
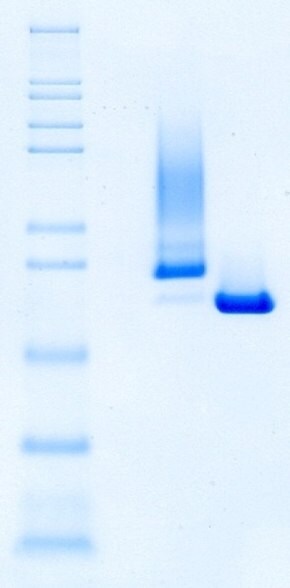
Figure 4.Identity and purity of Benzonase® Salt Tolerant Endonuclease and competitor A as shown by non-reducing SDS-PAGE.
The production in E.coli from Benzonase® Salt Tolerant endonuclease Emprove® Expert shows:
- Clean and pure protein
- Devoid of posttranslational modifications
- High batch to batch reproducibility
The conclusion in Figures 3 and 4 shows that Benzonase® Salt Tolerant endonuclease Emprove® Expert has a precisely defined molecular size. The benchmark endonuclease (Comp A) active at high salt does not.
Endonuclease Activity
Endonucleases rely on magnesium for their activity. Figure 5 provides a comparison of Benzonase® Salt Tolerant endonuclease and non-salt tolerant nucleases activity at different concentrations of magnesium. Benzonase® Salt Tolerant endonuclease activity was enhanced by high salt concentration and the activity was stable at a range of 1-10mM Mg2+ and 500mM salt. At 150mM and 500mM salt, Benzonase® salt tolerant endonuclease activity was similar to standard nuclease with 10mM Mg2+ (Figure 6).
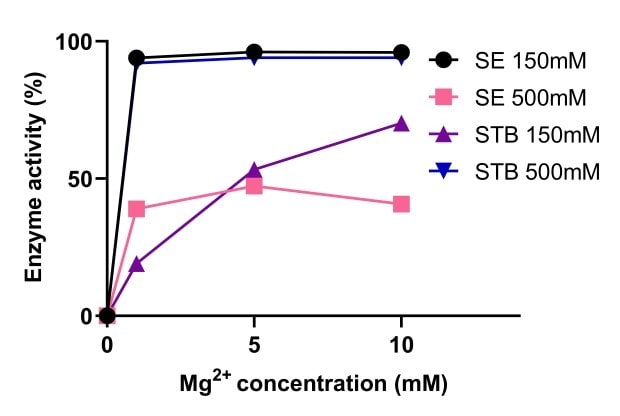
Figure 5.Comparison of the activity of Benzonase® Salt Tolerant endonuclease and a non-salt tolerant endonuclease.
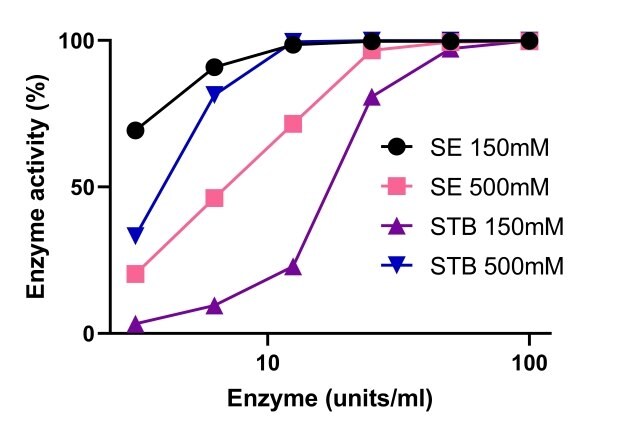
Figure 6.Comparison of the activity of Benzonase® Salt Tolerant endonuclease and a non-salt tolerant endonuclease.
Figure 7 shows the results of a study in which DNA was digested using Benzonase® Salt Tolerant endonuclease and other commercially available salt-active and non-salt-active endonucleases at both 500mM and 1M NaCl for one hour at 37 °C. Benzonase® Salt Tolerant endonuclease completely digested the DNA at 500mM and 1M salt and showed similar or better performance versus the other endonuclease.
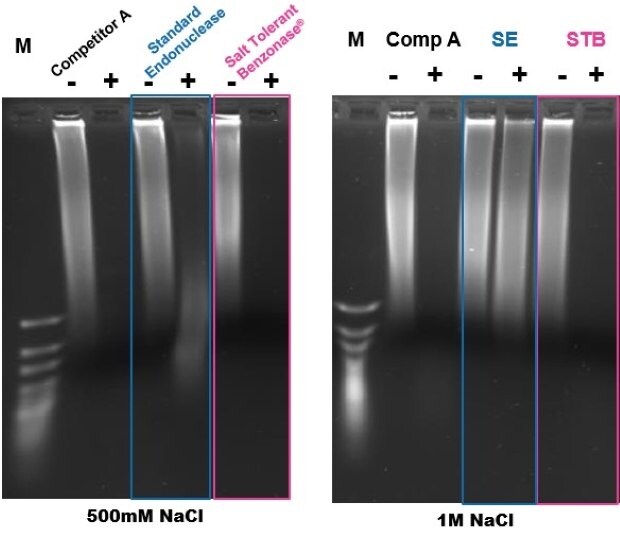
Figure 7.Comparison of DNA digestion with Benzonase® Salt Tolerant endonuclease and a commercially available salt-active and a non-salt-active endonuclease at 37°C for one hour.
Legend
M – GeneRuler Ultra Low Range DNA Ladder Cat# SM1213
Comp A ** – Salt Active endonuclease Competitor
SE – Standard endonuclease like Cat# 103773
STB – Benzonase® Salt Tolerant Endonuclease Emprove® Expert
Nucleic Acid Fragment Size
Benzonase® Salt Tolerant endonuclease digests DNA to undetectable levels below the size of 10 base pairs. Figure 8 shows DNA fragments run on a 4% agarose gel using a low molecular weight DNA marker. At 150mM salt, the DNA was not completely digested; at 500mM salt, no fragments of DNA remained following digestion.
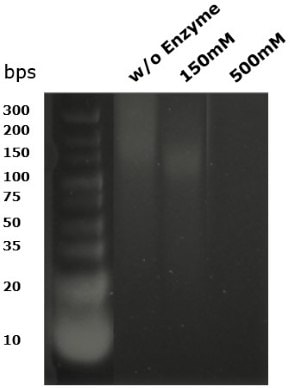
Figure 8.DNA fragment sized 25U/ml following digestion with Benzonase® Salt Tolerant endonuclease at 37°C for 30 minutes.
Compatibility of Benzonase® Salt Tolerant Endonuclease with Detergents in AAV Vector Production
The activity of Benzonase® Salt Tolerant endonuclease was evaluated when used in conjunction with four different detergents used for cell lysis: TRITON™ X-100, polysorbate 20, and two detergent alternatives developed in response to the TRITON™ X-100 ban – Deviron® C16 and 13-S9. As shown in Figure 9, the activity of Benzonase® Salt Tolerant endonuclease was not impacted by any of the four detergents. DNA was completely digested in the 500mM NaCl lysis buffer in the presence of TRITON™ X-100, polysorbate 20, and Deviron® 13-S9 (7B).
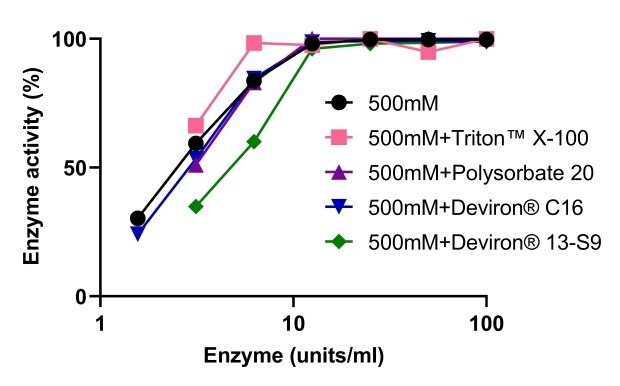
Figure 9.Benzonase® Salt Tolerant endonuclease activity when used in combination with different detergents.
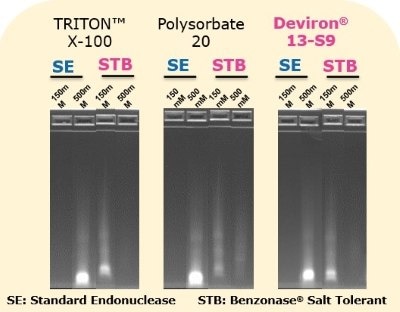
Figure 10.Benzonase® Salt Tolerant digestion of DNA when used in combination with different detergents.
Selecting the Right Benzonase® for Your Application
Benzonase® Salt Tolerant endonuclease should be used for applications in which the salt concentration is 200mM or higher. At a concentration of 500mM salt, DNA will be completely digested, and as shown above, it is possible to achieve a 29% increase in AAV titer and close to a 2,000% infectivity. Patient safety is increased with use of high salt conditions for this midstream step as AAV aggregation is hindered and effective removal of DNA from viral capsids is ensured. The post translational profile of the Benzonase® Salt Tolerant endonuclease also ensures the use of a non-glycosylated and easy to detect protein in drug production.
For applications in which the salt concentration is less than 200mM, Benzonase® Safety Plus standard endonuclease should be used.
Table 3 summarizes the specifications of our three GMP Benzonase® endonuclease references. Benzonase® Salt Tolerant endonuclease is part of the Emprove® program which helps manufacturers minimize disruptions and conduct thorough risk assessments when moving from development to manufacturing. The program provides raw and starting materials that are stringently qualified according to industry-leading standards. Materials that are part of the program are supported by comprehensive documentation packages that meet the information needs of pharmaceutical manufacturers when qualifying raw materials, completing a risk assessment, and optimizing a manufacturing process.
Solving the High Salt Challenge
Use of a high salt concentration during the cell lysis step in AAV production delivers higher titers and improved infectivity. Historically, use of this approach was impractical, however, as high salt conditions inhibit activity of conventional endonucleases used for DNA digestion.
Availability of Benzonase® Salt Tolerant endonuclease enables incorporation of salt concentrations on the order of 500mM during the midstream lysis and DNA digestion step and is compatible with all tested detergents including Deviron® detergents which can serve as alternatives to TRITON™ X-100.
To request a sample of either Benzonase® Salt Tolerant endonuclease or Deviron® portfolio product, please click the link below to fill out our very short webform.
Benzonase® FAQ
Definition
What is Benzonase® endonuclease?
Benzonase® endonuclease is a trade name owned by Merck for endonucleases used in research and bioprocessing applications.
What enzymes are found under this trade name?
The Benzonase® endonuclease family currently hosts two different enzymes.
- The legacy Benzonase® endonuclease product line, such as the Benzonase® endonuclease Safety Plus, isolated from Serratia marcescens, and recombinantly produced in E. coli K12 strain W3110. The protein has a molecule weight of 30 kDa and an isoelectric point (PI) of 6.85.
- The new Benzonase® Salt Tolerant endonuclease (1.4445). It was designed with state-of-the-art protein engineering capabilities, to ensure the highest activity at high salt concentrations.
The protein is a monomer with a molecular weight of about 27 kDa and a pI at pH 9.68. A dedicated section of this FAQ specifically covers this enzyme.
Mode of Action
What type of nucleic acids does Benzonase® Nuclease work on? Can I use it when isolating RNA?
Benzonase® Nuclease is a promiscuous endonuclease that attacks and degrades all forms of DNA and RNA (single stranded, double stranded, linear and circular).
What is the end result of complete nucleic acid degradation by Benzonase® nuclease?
Benzonase® nuclease completely digests nucleic acids to 5’-monophosphate terminated oligonucleotides that are 2‒5 bases in length.
Inhibition and Removal
How can Benzonase® Nuclease be inactivated? How can it be removed?
Reversible inhibition can be achieved using EDTA to chelate essential metal ions. Irreversible inactivation can only be accomplished with extreme conditions (100 mM NaOH at 70 °C for 30 minutes). Benzonase can be separated from the target product using chromatography. However, because of the robust nature of this endonuclease, we recommend that Benzonase not be used if a nuclease-free end product is required.
Why is Benzonase® endonuclease not working? What will inhibit its activity?
Benzonase® endonuclease is active under a wide range of operating conditions (see Enzyme Characteristics paragraph); however, a concentration of 1–2 mM Mg2+ is essential for the activity of Benzonase® endonuclease.
Mn2+ can substitute Mg2+; however, the enzyme will only reach its optimum activity in the presence of Mg2+. It is inhibited (approximately 50% activity) by monovalent cation concentrations > 300 mM, phosphate concentrations > 100 mM, and by ammonium sulfate concentrations > 100 mM. In addition, concentrations of > 1 mM EDTA will also inhibit Benzonase® endonuclease activity.
I observe a loss of activity: why?
Benzonase® endonuclease is usually very stable; however, in rare cases a loss of activity can be observed. There are several possible reasons for this: irreversible inactivation can be due to the presence of denaturing agents in the sample, e.g., proteases; or, alternatively, due to incorrect storage. Reversible inactivation is commonly due to the presence of chelating agents such as EDTA, which remove essential magnesium ions.
How do I remove Benzonase® endonuclease in a bioprocessing template?
Removal of Benzonase® endonuclease can be accomplished by several downstream units of operation, like depth filtration for clarification, tangential flow filtration (TFF) for concentration and diafiltration and chromatography (IEX, SEC, HIC). Please see Appendix, Chapter 2 “Removal of Benzonase® endonuclease” (page 36) for further information.
STABILITY AND OPERATING CONDITIONS
My Benzonase® endonuclease was left out on the bench. Is it still good?
We have done extensive stability testing on Benzonase® endonuclease, and find that it is extremely stable. Even with extended incubations at 37 °C, Benzonase® Endonuclease maintained > 90% activity for several months.
I want to use a different buffer. What conditions are absolutely required for full activity of Benzonase® endonuclease? What will reduce its activity?
Benzonase® endonuclease requires 1‒2 mM Mg2+ for activity. Benzonase is inhibited (approximately 50% activity) by monovalent cation concentrations >50%, phosphate concentrations >20 mM, and by ammonium sulfate concentrations >25 mM.
How much more Benzonase® endonuclease do I have to add if I am working at low temperatures?
Benzonase® endonuclease requires 1‒2 mM Mg2+ for activity. Benzonase is inhibited (approximately 50% activity) by monovalent cation concentrations >50%, phosphate concentrations >20 mM, and by ammonium sulfate concentrations >25 mM.
PROTEIN EXTRACTION APPLICATIONS
Is Benzonase® endnuclease compatible with protease inhibitor cocktails?
Yes. However, caution should be exercised, since many protease inhibitor cocktails include EDTA. Concentrations of greater than 1 mM EDTA will inhibit the activity of Benzonase® endonuclease.
My protein is insoluble and I need to perform purification under denaturing conditions. Will Benzonase® endonuclease still work in urea?
Benzonase® endonuclease activity actually increases in presence of urea at concentrations up to 6 M. At 6 M urea, enzyme activity first increases, then decreases over time. At 7 M urea, Benzonase® endonuclease denatures after 15 minutes, and activity is lost. However, significant degradation of nucleic acids occurs before inactivation. Higher initial concentrations of Benzonase® endonuclease can partially compensate the effects of 7 M urea.
Why do you have so many varieties of Benzonase® Endonuclease? What does HC mean? What impact does 90% versus 99% purity have?
To meet the widest possible range of processing and cost requirements, Benzonase® endonuclease is available in two different purity grades: Purity grade I (>99% pure) and Purity grade II (>90% pure). Both grades are available at 25 U/μL or at a high concentration (HC), which is defined as 250 U/μL. For bulk purchases, contact Custom Services.
For a complete list of Benzonase® products and the differences between them, visit our endonuclease page here.
What is the end result of complete nucleic acid degradation by Benzonase® endonuclease?
Benzonase® endonuclease completely digests nucleic acids to 5’-monophosphate terminated oligonucleotides that are 2‒5 bases in length.
BIOMANUFACTURING APPLICATIONS
Which quality/quantity of Benzonase® endonuclease will be adequate for a certain application?
There are several parameters that influence the activity of Benzonase® endonuclease. Hence, the optimum conditions will vary from process to process and need to be determined experimentally. For viscosity reduction, Benzonase® endonuclease, purity grade II (≥ 90%) will often be sufficient. Under standard assay conditions one unit of Benzonase® endonuclease corresponds approximately to the amount of enzyme required to completely digest 37 µg of DNA within 30 minutes.
At which step do I have to introduce Benzonase® endonuclease in my process?
The answer to this question will vary depending on why you are using Benzonase® endonuclease. The example applications given will hopefully help you answer this question. However, as a general rule, Benzonase® endonuclease is usually best added after the cultivation and before the capture step.
Is Benzonase® endonuclease safe?
Yes, toxicological studies with Benzonase® endonuclease have been performed (internal reports available). The systemic toxicity after single application was investigated in mice and rats: no toxic effects have been observed even at very high doses. In addition, no mutagenic potential has been observed in mice treated intravenously even with a very high dose of Benzonase® endonuclease.
Why is the filling range volume of the 5-million-unit tubes not specified?
As the activity (U/mL) of Benzonase® endonuclease may vary between production lots, we decided to specify the units per tube, but not the volume. The volume per tube can be easily calculated from the activity information on the Certificate of Analysis (CoA).
BENZONASE® SALT TOLERANT ENDONUCLEASE
Is the sequence similar to legacy Benzonase® products like the Benzonase® Safety Plus?
The Benzonase® Salt Tolerant Endonuclease is a brand-new enzyme designed through state-of-the-art protein engineering to ensure the highest activity at high salt concentration. It owns a different amino acid sequence in comparison to other Benzonase® legacy products.
What are the differences regarding operating conditions with legacy Benzonase® products?
As an endonuclease, the Benzonase® Salt Tolerant Endonuclease operates with the same conditions as legacy Benzonase® products. The exception is for monovalent cation concentration, where we recommend a minimum of 300mM NaCl.
How do I inhibit this enzyme?
The enzyme can be inhibited by addition of EDTA or exposure to heat.
How do I remove this enzyme?
Refer to “How do I remove Benzonase® endonuclease in a bioprocessing template?“ above.
How do I detect traces of this enzyme?
For our legacy Benzonase® enzyme, a dedicated Elisa kit is completing our offer to ensure a robust detection of Benzonase® Salt Tolerant endonuclease.
Why should I use this enzyme instead of other standard nucleases?
All standard nucleases used for bioprocessing on the market are based on a very similar amino acid sequence. It means their ability to digest nucleic acids at high salt is comparable and considered not optimal.
Their effective operating conditions for monovalent cation concentrations ranges between 0 and 200mM. Similar to physiological salt concentrations found in biological processes.
In parallel, it has been shown that the use of higher salt concentrations, above phycological concentration during HEK cell lysis, is having a real impact on the overall process yield. At 500mM NaCl concentrations, the infectivity and overall AAV recovery rate is significantly higher than at 150mM salt concentration.
The use of a salt tolerant nuclease is then necessary to remove nucleic acid in these high salt conditions.
Can I still use regular nucleases at salt concentrations >200mM?
Activity of regular nucleases at higher than physiological salt concentrations will be drastically reduced, leading to a need to add more enzymes and complexifying the drug purification process. This also leads to a poor cost-effective process with higher costs of ownership. For that reason, we recommend the use of a salt tolerant nuclease.
Can I use detection kits from other suppliers to detect the Benzonase® Salt Tolerant Endonuclease? Like other kits specialized for other “salt active” nucleases?
The Benzonase® Salt Tolerant Endonuclease is a brand-new enzyme, no detection kit can detect it on the market outside our own detection kits.
Note: Any product not sold via an official Merck channel, where the trade name “Benzonase®” is used, should be considered as a counterfeit product.
Why is the expression host so important for enzyme production?
Protein expression can be achieved with multiple production platforms. Yeast based and Bacterial based expressions are the most common. Still, resulting products are not similar and post translational modification profiles are different depending on the platform used.
Yeast Expressed proteins are heavily glycosylated and modified which can lead to various issues during product usage such as a poor detection accuracy with antibody assays.
The main Salt Active Endonuclease on the market is expressed in Yeast.
Why can post translational modification of nucleases impact the detection accuracy and efficiency?
Glycosylated proteins are showing a non-homogeneous profile (as seen in figure 2), not reproducible from batch to batch. As standard detection methods are using immunoassays, antibodies created to detect the protein of interest cannot cover the full spectrum of modifications observed with yeast expression. This leads to a poor detection accuracy of antibody-based detection methods such as Elisa Kits.
What is the toxicological profile of this enzyme?
The Benzonase® Salt Tolerant endonuclease comes with the same toxicological profile as other members of the Benzonase® portfolio.
Can you help me define which nuclease would be the most cost effective for my process? Keeping in mind overall process efficiency and yield.
We have an entire portfolio of nucleases meeting your regulatory and process condition needs. Do not hesitate to request our MSAT (Manufacturing Science And Technology) support for your process development. Our experts cover full manufacturing templates, from Upstream to Final Fill.
Deviron® FAQ
Can I keep using TRITON™ X-100 if I’m not located in the European Union?
The TRITON™ X-100 ban has been decided by the ECHA1 under the REACH2 regulation. The main driver was the high toxicity of the product for human health and environment. Therefore, removing this product from bioproduction is one of the hottest trends in the industry. Non-E-U regulatory agencies will also evaluate the product usage, leading to other bans to come.
Why can’t I use a research grade detergent for biomanufacturing?
Detergents are in general manufactured in huge volumes with organic chemistry chain reactions. Often the raw material quality is poor to drive costs of production down. This leads to very dangerous impurities in the end-product like dioxin or nitrosamine. These research grade detergents are used for cleaning applications only and should not be found close to drug formulation.
What are the key steps to switch to Deviron®?
The Deviron® portfolio comes with 3 batches available of GMP EXCiPACT manufactured product. Samples of these batches can be already requested for qualification purpose. The Emprove® dossiers coming with our products contains all information needed to file to regulatory agencies. Toxicology information are also readily available.
I want to try out Deviron® but I have no expertise, how can you support me?
For application related support, you can rely on our team of Biomanufacturing experts to guide you through this change. Get in touch with your Merck contact to get free support.
What is the difference between MQ400/Emprove® Evolve and MQ500/Emprove® Expert (GMP)?
To help with your regulatory filling, we created different documentation and quality programs to help you get the right product for the right application. Click on the following links to learn more about the Emprove® and M-Clarity® programs. The Deviron® C16 Emprove® Evolve and the Deviron® 13-S9 Emprove® Expert are part of the highest quality levels we offer.
What are the recommended concentrations for target applications?
For Viral inactivation, the ASTM3 E3042-16 used for TRITON™ X-100 is still as of today the document of choice for Monoclonal antibodies manufacturing. Concentrations used in the industry vary between 0,5 and 1% based on the application.
For cell lysis applications, a wide range of concentrations are used, all process specific. We would recommend trying several concentrations in parallel during process development. Our Deviron® detergents are at least as efficient as TRITON™ X-100 or Polysorbates.
Can we develop a GMP detection method for Deviron®?
Our R&D experts developed detection methods for our Deviron® portfolio that can be transferred to our customers. However, for GMP detection methods, our Bioreliance® laboratory is the partner of choice. Get in touch with us for any question.
Is the Deviron® portfolio compatible with Benzonase® for cell lysis applications?
Benzonase® is the gold Standard for DNA digestion in AAV4 processes. The Deviron® portfolio has been developed to be fully compatible with Benzonase® Endonucleases. There is no loss of enzymatic activity or detergent property with the use of the two portfolios in combination. Application data can be found in the Deviron® portfolio brochure.
What is the manufacturing capacity of the Deviron® portfolio?
The Deviron® portfolio is released out of our Darmstadt site in Germany, we can already offer more than 100 tons of product per year. Do not hesitate to provide us with a forecast to discuss case specific lead-times.
1European Chemicals Agency
2Registration, Evaluation, Authorization and Restriction of Chemicals*
3American Standard for Test Method
4Adeno Associated Virus
TRITON is a trademark of The Dow Chemical Company (“Dow”) or an affiliated company of Dow, used under license.
For more detailed information on the AAV Process Intensification Using High Salt Lysis and Salt Tolerant Endonuclease:
- Read the data sheet "Benzonase® Salt Tolerant endonuclease Emprove® Expert"
- See the the technical poster "AAV process intensification using high salt lysis & Benzonase® Salt Tolerant endonuclease"
- See the family portfolio flyer "Explore the Benzonase® endonuclease Portfolio"
- See the Deviron® detergent portfolio brochure "Explore Greener Alternatives to Triton™ X-100 for Biopharmaceutical Applications"
References
We provide information and advice to our customers to the best of our knowledge and ability, but without obligation or liability. Existing laws and regulations are to be observed in all cases by our customers. This also applies in respect to any rights of third parties. Our information and advice do not relieve our customers of their own responsibility for checking the suitability of our products for the envisaged purpose.
To continue reading please sign in or create an account.
Don't Have An Account?