Select a Size
About This Item
Product Name
Tetrakis(triphenylphosphine)palladium(0), 99%
InChI key
NFHFRUOZVGFOOS-UHFFFAOYSA-N
InChI
1S/4C18H15P.Pd/c4*1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;/h4*1-15H;
SMILES string
[Pd].c1ccc(cc1)P(c2ccccc2)c3ccccc3.c4ccc(cc4)P(c5ccccc5)c6ccccc6.c7ccc(cc7)P(c8ccccc8)c9ccccc9.c%10ccc(cc%10)P(c%11ccccc%11)c%12ccccc%12
assay
99%
form
solid
reaction suitability
core: palladium, reaction type: Cross Couplings, reaction type: Heck Reaction, reaction type: Hiyama Coupling, reaction type: Negishi Coupling, reaction type: Sonogashira Coupling, reaction type: Stille Coupling, reaction type: Suzuki-Miyaura Coupling, reagent type: catalyst
reaction type: Buchwald-Hartwig Cross Coupling Reaction
parameter
air sensitive
storage temp.
2-8°C
Quality Level
Looking for similar products? Visit Product Comparison Guide
General description
Tetrakis(triphenylphosphine)palladium(0) (Pd(PPh3)4) is a commonly used palladium(0) complex in chemical synthesis. It plays a vital role as a catalyst in various organic reactions, especially C-C, C-N, C-O, and C-heteroatom cross-coupling reactions. Pd(PPh3)4 finds extensive application in carbon-carbon bond-forming reactions such as the famous Heck, Suzuki-Miyaura, and Stille reactions. These reactions involve the coupling of aryl, alkyl, or vinyl halides with other organic compounds to yield valuable products. The catalytic cycle involves the oxidative addition of the organic halide to palladium(0), transmetalation with a suitable organometallic reagent or boronic acid, and reductive elimination to form the desired product. Pd(PPh3)4 has also been utilized in various other transformations including allylic substitutions, nucleophilic additions to alkenes and alkynes, and cycloadditions.
Application
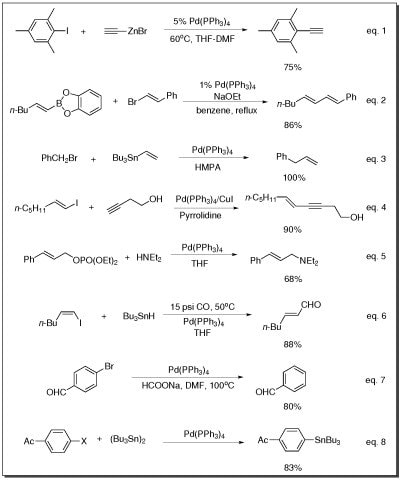
- Negishi coupling (eq. 1), Suzuki coupling (eq. 2), Stille coupling (eq. 3), Sonogashira coupling reaction (eq. 4), and Buchwald-Hartwig amination reaction (eq. 5)
- The carbonylation of vinyl iodides (eq. 6)
- The reduction reaction of aryl bromides (eq. 7)
- Carbon-tin bond formation (eq. 8)
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Which document(s) contains shelf-life or expiration date information for a given product?
If available for a given product, the recommended re-test date or the expiration date can be found on the Certificate of Analysis.
How do I get lot-specific information or a Certificate of Analysis?
The lot specific COA document can be found by entering the lot number above under the "Documents" section.
The color of Product 216666, Pd tetrakis(triphenylphosphine)Cl was a yellow or gold color, but now my material has turned a tan, brown, or green color. Is it still good to use?
A change in color of the material indicates oxidation and it will no longer be active as a catalyst.
How do I find price and availability?
There are several ways to find pricing and availability for our products. Once you log onto our website, you will find the price and availability displayed on the product detail page. You can contact any of our Customer Sales and Service offices to receive a quote. USA customers: 1-800-325-3010 or view local office numbers.
What is the Department of Transportation shipping information for this product?
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
My question is not addressed here, how can I contact Technical Service for assistance?
Ask a Scientist here.
Articles
Effective in key synthesis reactions like Knoevenagel condensation, thalidomide synthesis, and Suzuki coupling for sustainable chemical transformations.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service